Factor Xa inhibitor effective and safe in AF patients with liver disease
Edoxaban Versus Warfarin in Patients With Atrial Fibrillation and History of Liver Disease
Literature - Qamar A, Antman EM, Ruff CT et al. - J Am Coll Cardiol 2019;74:179–89, doi.org/10.1016/j.jacc.2019.04.061Introduction and methods
Liver disease is associated with increased risk of atrial fibrillation (AF) [1,2] and AF patients with liver disease require anticoagulation. However, there is an increased risk of bleeding in these patients due to decreased production of coagulation factors, thrombocytopenia, and increased fibrinolysis [3]. Randomized clinical trials examining direct oral anticoagulant agents (DOACs) often exclude patients with liver disease [4-6]. In addition, DOACs are metabolized by the hepatobiliary system and therefore patients with liver disease may have different drug levels, thereby affecting anticoagulation and bleeding risk.
Clearance of the direct oral factor Xa (FXa) inhibitor edoxaban is mediated by the liver (up to 50%) and the kidneys (50%) [7]. In the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis In Myocardial Infarction Study 48), the higher-dose edoxaban was noninferior to warfarin in preventing the primary endpoint of stroke or systemic embolic events (SSEE) and was associated with significantly lower rates of major bleeding, intracranial hemorrhage, and CV mortality [8]. Based on these results, edoxaban was approved by the FDA for the prevention of stroke or systemic embolism in AF patients.
There have been small studies on edoxaban and other DOAcs in patients with liver disease [9-12] and based on their results, the FDA recommends use of edoxaban without dose adjustment in patients with mild hepatic impairment and contradicts using it in patients with moderate of severe hepatic impairment.
In this analysis of the ENGAGE AF-TIMI 48 study, pharmacokinetics, pharmacodynamics, efficacy and safety of edoxaban vs. warfarin in AF patients with and without liver disease were examined. ENGAGE AF-TIMI 48 enrolled 21,105 patients ≥21 years with AF and a CHADS2 score ≥2. History of liver disease was determined by the site investigator, or determined by elevated liver enzymes at randomization. Patients were assigned in a 1:1:1 ratio to receive high-dose edoxaban, lower-dose edoxaban or warfarin. Median follow-up was 2.8 years.
Main results
- Of 21,105 patients enrolled in ENGAGE AF-TIMI 48, 1,083 (5.1%) had a history of liver disease.
- There was no difference between patients with liver disease compared to patients without liver disease for the risk of SSEE, ischemic SSEE or hemorrhagic stroke. Nor were all-cause death, CV death and myocardial infarction different between groups.
- Risk of ISTH major bleeding was higher in patients with liver disease than in those without (adjHR: 1.38, 95%CI: 1.10-1.74, P=0.006), as well major or clinically relevant nonmajor bleeding. Intracranial hemorrhage and fatal bleeding were not different between the groups.
- There were no significant differences in PK and PD assessment of edoxaban in patients with liver disease compared to those without.
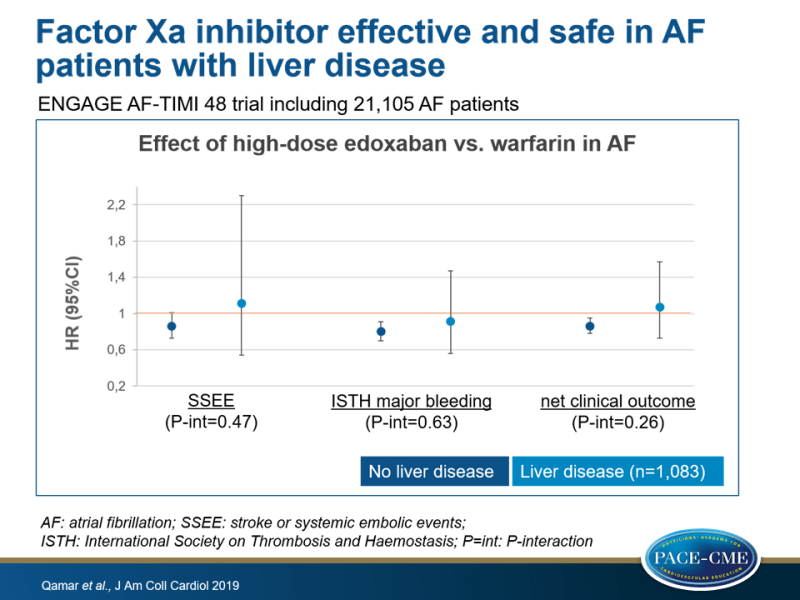
- Annualized rate of SSEE was similar with high-dose edoxaban vs. warfarin in patients without liver disease (HR: 0.86; 95%CI: 0.73-1.01) compared to patient with liver disease (HR: 1.11, 95%CI:0.54-2.30) (P-interaction=0.47). There were also no interactions for the individual components ischemic stroke, hemorrhagic stroke, systemic embolic event or all-cause death.
- Annualized rate of ISTH major bleeding was similar with high-dose edoxaban vs. warfarin in patients without liver disease (HR: 0.80, 95%CI:0.70-0.91) compared to those with liver disease (HR: 0.91, 95%CI:0.56-1.47) (P-interaction=0.63). No significant interaction were seen for fatal bleeding and clinically relevant nonmajor bleeding or major bleeding by presence of liver disease.
- Primary net clinical outcome, a composite of SSEE, major bleeding or death by any cause for high-dose edoxaban vs. warfarin was not different between patients without liver disease (HR: 0.86) or with liver disease (HR: 1.07) (P-interaction=0.26).
- There were no significant differences in rates of severe, moderate, mild or minimal liver injury between low-dose edoxaban, high-dose edoxaban and warfarin groups.
Conclusion
In this analysis of the ENGAGE AF-TIMI 48 study, in AF patients with liver disease risk of thrombotic events was not increased, but risk of bleeding was increased when compared to AF patients without liver disease. Effectiveness and safety of high-dose edoxaban compared to warfarin was similar in patients with liver disease compared to those without. PK and PD profiles of edoxaban were similar in patients with and without liver disease and hepatic adverse events were similar in groups.
References
1. Wijarnpreecha K, Boonpheng B, Thongprayoon C, Jaruvongvanich V, Ungprasert P. The association between non-alcoholic fatty liver disease and atrial fibrillation: a meta-analysis. Clin Res Hepatol Gastroenterol 2017;41:525–32.
2. Alonso A, Misialek JR, Amiin MA, et al. Circulating levels of liver enzymes and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities cohort. Heart 2014;100:1511–6.
3. Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol 2018;71:2162–75.
4. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51.
5. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92.
6. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91.
7. Parasrampuria DA, Truitt KE. Pharmacokinetics and pharmacodynamics of edoxaban, a nonvitamin K antagonist oral anticoagulant that inhibits clotting factor Xa. Clin Pharmacokinet 2016; 55:641–55.
8. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104.
9. Mendell J, Johnson L, Chen S. An open-label, phase 1 study to evaluate the effects of hepatic impairment on edoxaban pharmacokinetics and pharmacodynamics. J Clin Pharmacol 2015;55:
1395–405.
10. Stangier J, Stahle H, Rathgen K, Roth W, Shakeri-Nejad K. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol 2008;48:1411–9.
11. Kubitza D, Roth A, Becka M, et al. Effect of hepatic impairment on the pharmacokinetics and
pharmacodynamics of a single dose of rivaroxaban, an oral, direct Factor Xa inhibitor. Brit J Clin Pharmacol 2013;76:89–98.
12. Frost CE, Yu Z, Wang J, et al. Single-dose safety and pharmacokinetics of apixaban in subjects with mild or moderate hepatic impairment (abstr). Clin Pharmacol Ther 2009;85 Suppl 1. S34 (PI-84).
Find this article online at JACC

Facebook Comments