First time a CETP inhibitor shows reduction of serious CV events
ESC 2017 - Barcelona
News - Aug. 29, 2017Anacetrapib reduces risk of serious cardiovascular events in high risk patients on statins (REVEAL)
Presented at the ESC congress 2017 by: Martin Landray (Oxford, UK)
Background
Clinical trials with cholesteryl ester transfer protein (CETP) inhibitors, which increase HDL-c, have not shown a CV benefit to date. These included trials with torcetrapib (unexpectedly high cardiovascular mortality), dalcetrapib or evacetrapib (lack of efficacy). Anacetrapib is a new CETP inhibitor that was evaluated in the REVEAL study. This study included many more patients and had a longer follow-up than earlier published studies evaluating CETP inhibitors. 30449 patients of at least 50 years old with vascular disease and total cholesterol levels of <155 mg/dL (<4.0 mmol/L) participated in the REVEAL study and received 100 mg anacetrapib or placebo daily, on top of atorvastatine (20 or 80 mg daily).
Main results
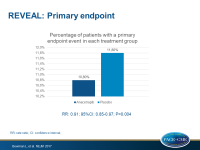
- During a median of 4.1 years of follow-up 10.8% of anacetrapib-treated patients and 11.8% of placebo-treated patients experienced a primary endpoint event (coronary death, myocardial infarction (MI) or coronary revascularization). This translated into a significant 9% proportional reduction in major coronary events (HR 0.91, 95% CI 0.85-0.97, P=0.004). Kaplan-Meier analyses showed that the curves diverged after 2 years.
- The components of the primary outcome were all significantly reduced in the anacetrapib group, with the exception of a non-significant reduction of coronary death (HR 0.92, 95% CI 0.80-1.06, P=0.25) (coronary revascularisation: HR 0.90, 95% CI 0.83-0.97, P=0.01, MI: HR 0.87, 95% CI 0.78-0.96, P=0.007 and coronary death and MI: HR 0.89, 95% CI 0.81-0.97, P=0.008.
- Median increase of HDL with anacetrapib was 43 mg/dL (doubling).
- Median reduction of non-HDL with anacetrapib was 17 mg/dL.
- LDL-c was well regulated during the trial: median 1.6 mmol/L (20% reduction).
- Both systolic and diastolic blood pressure increased slightly, but this did not translate into an increase of serious hypertension. A surprising decrease of new onset diabetes mellitus (DM) was seen with anacetrapib (5.3% vs 6.0% on placebo, P=0.05). Concerning renal disease, an increase of new onset eGFR <60 mL/min/1.72 m2 was seen with anacetrapib (11.5% vs 10.6% on placebo, P=0.04).
- No safety signals were seen with regard to vascular, non-vascular death, or death by any cause, nor with regard to incidence of cancer, and complications relating tot he liver, muscles and cognitive function.
Conclusion
Treatment with the CETP inhibitor anacetrapib on top of statin therapy resulted in a significant reduction of serious coronary events compared with placebo, in high-risk patients with vascular disease. A small reduction in the number of persons with new onset DM was seen. Anacetrapib was well tolerated, without an excess of symptomatic complications or serious side-effects, considering absence of an excess of symptomatic complications and serious side-effects. A post-trial follow-up is planned in which patients will be followed for two extra years, while not taking anacetrapib anymore, to assess the efficacy and safety of anacetrapib in the long run.
Discussion
During the press conference it was noted that the Kaplan-Meier curves diverged only after 2 years. Landray noted that this is comparable to other lipid-lowering studies. It is possible that other CETP inhibitor studies were too short to achieve an effect.
During the press conference, the attendants speculated about the mechanism through which anacetrapib might reduce the number of serious coronary events; possibly the effects on non-HDL play a role. Landray noted that an event reduction of 9% cannot be explained by only a doubling of HDL-c levels. Thus there must be other elements that lead to this, maybe the strongly decreased non-HDL-c. This reduction matches the curve of risk reduction of coronary death and MI events seen in LDL-c lowering statin studies. This hypothesis is corroborated by genetic studies that show that CETP-related variants that are associated with a lower coronary risk, are mostly associated with lower LDL. Thus, it appears as though non-HDL is the main cause of the observed effect of anacetrapib.
Finally, the clinical relevance of this 9% reduction of events was discussed. Landray emphasized the size of the study and the number of events despite maximal therapy. He considers this 9% reduction relevant for patients.
- Our reporting is based on the information provided at the ESC congress -







Facebook Comments