Primary endpoint just missed significance in trial evaluating ARNI in HFpEF patients
Introduction and methods
News - Sep. 1, 2019Angiotensin Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction: The PARAGON-HF Trial
Presented at ESC Congress 2019 in Paris, France by Scott Solomon (Boston, MA, USA)
Up to 64 million people worldwide have heart failure (HF), and the prevalence is rising with the aging population. There is only approved evidence-based treatment for patients with reduced ejection fraction (HFrEF, EF≤40%). Patients with HF with preserved EF (HFpEF) are hospitalized at nearly the same rate as those with lower EF. Thus, this condition represents a large unmet need.
The PARADIGM-HF trial has previously shown that the angiotensin neprilysin inhibitor (ARNI) sacubitril/valsartan reduced CV death and hospitalization for HF in patients with HFrEF. In a phase II trial in HFpEF, sacubitril/valsartan lowered NT-proBNP, improved left atrial size and New York Heart Association (NYHA) functional class, when compared with valsartan.
The PARAGON-HF study was a randomized, double-blind, active comparator study that tested the hypothesis that sacubitril/valsartan, compared to valsartan alone, would reduce the composite outcome of total HF hospitalizations and CV death. After an active run-in period in which patients of at least 50 years of age and LVEF ≥45% were exposed to valsartan 80 mg BID and subsequently to sacubitril/valsartan 49/51 mg BID, patients were randomized to sacubitril/valsartan 97/103 mg BID or valsartan 160 mg BID, on top of optimal background medications for comorbidities (excluding ACEi and ARB). Patients had signs and symptoms of HF (NYHA II-IV), structural heart disease confirmed on echo, and mild elevation in natriuretic peptides. They were not actively decompensated and had not other reasons for symptoms. The primary endpoint was the composite of total (first and recurrent) HF hospitalizations and CV death.
Main results
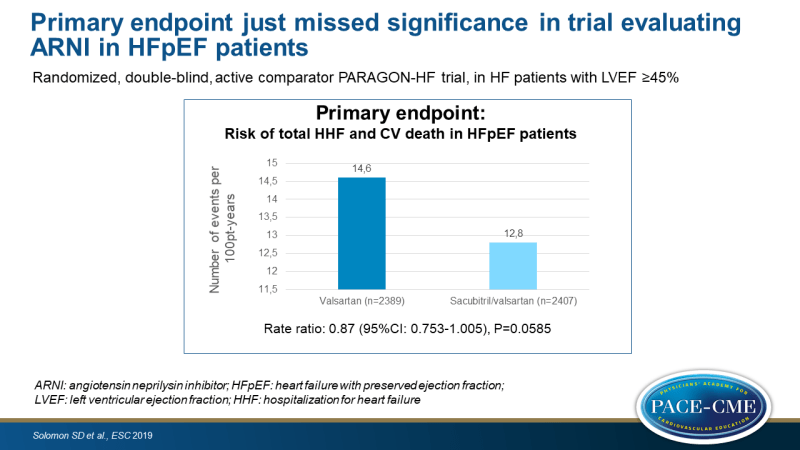
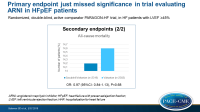
- The primary composite endpoint just missed significance, showing an event-rate with sacubitril/valsartan of 12.8 per 100 patient-years (PY), as compared with 14.6 per 100 PY, giving a rate ratio of 0.87 (95%CI: 0.753 – 1.005, P=0.0585).
- In sacubitril/valsartan-treated patients, 15.0% had improved NYHA functional class at 8 months as compared to baseline, while in 76.3% it was unchanged and in 8.7% it was worsened. In valsartan-treated patients, these percentages were 12.6%, 77.9% and 9.6%, respectively. This yielded an OR for improvement of 1.45 (95%CI: 1.13-1.86, P=0.0035).
- More sacubitril/valsartan-treated patients were KCCQ responders (greater than 5 points improvement), namely 33.0% vs. 29.6% with valsartan (OR: 1.30, 95%CI: 1.04-1.61, P=0.019).
- Fewer patients in the sacubitril/valsartan group had worsening renal function (1.4% vs. 2.7%, HR: 0.50, 95%CI: 0.33-0.77, P=0.002).
- No difference was seen in all-cause mortality (14.2% vs. 14.6%, HR: 0.97, 95%CI: 0.84-1.13, P=0.68).
- In a subgroup analysis based on sex, female patients (RR: 0.73, 95%CI: 0.59-0.90) seemed to benefit more from sacubitril/valsartan than male patients (RR: 1.03, 95%CI: 0.85-1.25).
- Similarly, a subgroup analysis showed that those with LVEF ≤ median (57%) showed greater benefit (RR: 0.78 95%CI: 0.64-0.95) than those with LVEF > median (RR: 1.00, 95%CI: 0.81-1.23).
Conclusions
The overall population of HFpEF patients showed a non-significant 13% reduction with sacubitril/valsartan treatment in the primary endpoint. Several supportive analyses and secondary endpoints, including how patients feel and kidney function, are suggestive of benefit with sacubitril/valsartan when compared with valsartan. The treatment effect, however, was heterogeneous, as a greater benefit was seen in patients with EF below normal, but not in the range normally considered reduced. Moreover, women showed greater benefit than men. These two groups showed an absolute risk reduction similar to that seen in patients with EF < 40%.
Safety and tolerability data were not shown during the press conference, but it was said that they were similar to in PARADIGM-HF, with more hypotension, but less elevation in serum creatinine and potassium with sacubitril/valsartan compared with valsartan.
These findings suggest that some patients with HFpEF, for whom no evidence-based therapy is available, may benefit from treatment with sacubitril/valsartan, particularly those with EF below normal but not truly reduced.
Discussion
During the press conference, dr. Solomon was asked whether he would use sacubitril/valsartan in the borderline group with EF below normal. He answered that the data show that these patients benefit, with a quite high absolute and relative benefit. Hence, in this group there is a rationale to use this agent. John McMurray, who was on the press conference panel, agreed. He said that he is normally very careful about interpretation of subgroup analyses. But it is not the first time they have seen that a drug that works in HFrEF, also works in those with low-normal EF. These people have mild systolic dysfunction. Experience from other trials suggests that may they have something to gain from probably all of these agents. We should remember that 40% is an artificial cut-off point: nothing changes biologically between EF of 39% and 40%. The difference between the effect in men and women was not discussed.
Several members on the panel considered this an important advance in the field, as the unmet need in patients with HFpEF is great. It was also mentioned that the cut-off point of 0.05 for statistical significance is artificial, which is the reason why more often people move to other types of analyses.
Moreover, a question was raised on how sacubitril/valsartan performed in black patients, as they have a higher risk of angioedema. The overall number of black patients was small, but in the United States, 25% of recruited patients were black. Sacubitril/valsartan indeed was more likely to cause angioedema (14 vs. 4%), but no life-threatening situations occurred.
- Our reporting is based on the information provided at the ESC congress -


Facebook Comments