Anacetrapib: a new approach to cardiovascular risk reduction
Anacetrapib, a novel CETP inhibitor: pursuing a new approach to cardiovascular risk reduction.
Literature - Gutstein DE et al; Clin Pharmacol Ther. 2012 Jan;91(1):109-22.Gutstein DE, Krishna R, Johns D, Surks HK, Dansky HM, Shah S, Mitchel YB, Arena J, Wagner JA.
Clin Pharmacol Ther. 2012 Jan;91(1):109-22. doi: 10.1038/clpt.2011.271.
Abstract
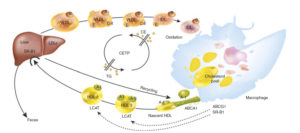
Cholesteryl ester transfer protein (CETP) inhibition is a promising experimental strategy to raise high-density lipoprotein cholesterol (HDL-C) and reduce cardiovascular risk. This review focuses on the highly selective and potent CE TP inhibitor anacetrapib and discusses the available preclinical and clinical information pertaining to it. We also describe strategies to target HDL-C, discuss the mechanism underlying CETP inhibition and its effects on lipid biology, and give an overview of other CETP inhibitors that are currently in development.
Rationale
As current LDL-C reducing strategies only lead to a ~30% reduction in the risk of major cardiovascular events [1], raising HDL-C might be a promising intervention, for example by inhibition of cholesterol ester transfer protein (CETP).Anacetrapib (N=808) n(%) | Placebo (N=804) n(%) | |
Prespecified adjudicated cardiovascular safety end point | 15 (1.9) | 21 (2.6) |
Cardiovascular death | 3 (0.4) | 1 (0.1) |
Nonfatal myocardial infarction | 6 (0.7) | 9 (1.1) |
Unstable angina | 1 (0.1) | 6 (0.7) |
Nonfatal stroke | 5 (0.6) | 5 (0.6) |
Total mortality | 11 (1.4) | 8 (1.0) |
Heart failure | 2 (0.2) | 4 (0.5) |
Revascularization | 8 (1.0) | 28 (3.5) |
Percutaneous coronary intervention | 6 (0.7) | 25 (3.1) |
Coronary artery bypass graft | 2 (0.2) | 3 (0.4) |
Conclusion
CETP inhibition is a promising target for improving cardiovascular outcomes in patients with dyslipidemia by lowering HDL-C. The outcomes of REVEAL are awaited to evaluate the usefulness of anacetrapib to reduce residual risk and to confirm the hypothesis that raising HDL-C results into cardiovascular benefit in patients with dyslipidemia and patients with established atherosclerotic heart disease.
References
1. Baigent, C.et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–1278 (2005).
2. Duffy, D. & Rader, D.J. Emerging therapies targeting high-density lipoprotein metabolism and reverse cholesterol transport. Circulation 113, 1140–1150 (2006).
3. Smith, C.J.et al. Biphenyl-substituted oxazolidinones as cholesteryl ester transfer protein inhibitors: modifications of the oxazolidinone ring leading to the discovery of anacetrapib. J. Med. Chem. 54, 4880–4895 (2011).
4. Cannon, C.P. et al.; Determining the Efficacy and Tolerability Investigators. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 363, 2406–2415 (2010).

Facebook Comments