Apixaban meets main goal of VTE study
12/12/2012
N Engl J Med. Dec 2012 . AMPLIFY-EXT study evaluated the use of apixaban for extended treatment of VTE
AbstractLiterature - Agnelli G, Buller HR et al. N Engl J Med. 2012 Dec 8
Apixaban for Extended Treatment of Venous Thromboembolism.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI; the AMPLIFY-EXT Investigators.N Engl J Med. 2012 Dec 8. [Epub]
Background
Apixaban is an oral factor Xa inhibitor that is administered in fixed doses without the need for laboratory monitoring. At a dose of 5 mg twice daily, apixaban has been shown to be effective for the prevention of stroke in patients with atrial fibrillation, and at a dose of 2.5 mg twice daily, the lowest dose evaluated in phase 2 studies, it has been shown to be effective for thromboprophylaxis after major orthopedic surgery [1-3]. AMPLIFY-EXT (Apixaban after the initial Management of PuLmonary embolIsm and deep vein thrombosis with First-line therapY-EXTended Treatment), a randomized, double-blind, multicenter trial, included 2,486 patients with prior VTE who had completed 6 to 12 months of anticoagulation treatment for DVT or PE and for whom there was clinical equipoise about the need for continued anticoagulation. Patients were randomized to receive either apixaban 2.5 mg or 5 mg, or placebo twice daily for 12 months. The primary efficacy outcome was the combined endpoint of symptomatic, recurrent VTE (nonfatal DVT or nonfatal PE) and death from any cause. Death was classified as VTE-related, cardiovascular-related, due to bleeding, or due to other causes.
Main results
- One year of treatment with apixaban 2.5 and 5 mg bid was superior to placebo in the reduction of the composite endpoint of symptomatic, recurrent VTE and death from any cause (11.6% in the placebo group, compared with 3.8% and 4.2% in the 2.5 mg and 5 mg groups, respectively, P<0.001), the primary efficacy outcome of the trial.
- Apixaban was superior to placebo for the predefined secondary efficacy outcome of recurrent VTE and VTE-related death (8.8% in the placebo group, compared with 1.7% in both the apixaban 2.5 mg and 5 mg groups). Both of these endpoints, the primary and secondary efficacy outcomes, were statistically significant (p<0.001) (fig. 1).
- The rate of the primary safety outcome of major bleeding was comparable across treatment groups (0.2 % for apixaban 2.5 mg; 0.1 % for apixaban 5 mg and 0.5% for placebo). The rate of the composite of major bleeding and clinically relevant non-major bleeding for the 5 mg treatment group (4.3%) was higher versus the placebo group (2.7%), while the rate for the 2.5 mg treatment group (3.2%) was similar to the placebo group (fig. 2).
Conclusion
For patients with venous thromboembolism for whom there is uncertainty about the benefits and risks of continued therapy, the results of this study provide a rationale for continuing anticoagulation therapy for an additional 12 months.Additional studies will be needed to determine the potential net benefit and risk of extending treatment beyond 18 to 24 months.
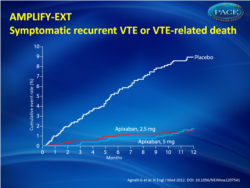 Figure 1.Kaplan–Meier cumulative event rates for the composite secondary efficacy outcome of symptomatic recurrent venous thromboembolism (VTE) or VTE-related death. |
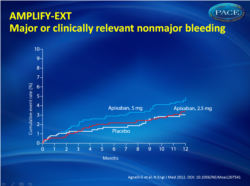 Figure 2.Kaplan–Meier cumulative event rates for the secondary safety outcome of the composite of major or clinically relevant nonmajor bleeding |
References
1. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92.2. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-17.
3. Raskob GE, Gallus AS, Pineo GF, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip or knee replacement: pooled analysis of major venous thromboembolism and bleeding in 8464 patients from the ADVANCE-2 and ADVANCE-3 trials. J Bone Joint Surg Br 2012;94:257-64.
Background
Apixaban, an oral factor Xa inhibitor that can be administered in a simple, fixed-dose regimen, may be an option for the extended treatment of venous thromboembolism.
Methods
In this randomized, double-blind study, we compared two doses of apixaban (2.5 mg and 5 mg, twice daily) with placebo in patients with venous thromboembolism who had completed 6 to 12 months of anticoagulation therapy and for whom there was clinical equipoise regarding the continuation or cessation of anticoagulation therapy. The study drugs were administered for 12 months.
Results
A total of 2486 patients underwent randomization, of whom 2482 were included in the intention-to-treat analyses. Symptomatic recurrent venous thromboembolism or death from venous thromboembolism occurred in 73 of the 829 patients (8.8%) who were receiving placebo, as compared with 14 of the 840 patients (1.7%) who were receiving 2.5 mg of apixaban (a difference of 7.2 percentage points; 95% confidence interval [CI], 5.0 to 9.3) and 14 of the 813 patients (1.7%) who were receiving 5 mg of apixaban (a difference of 7.0 percentage points; 95% CI, 4.9 to 9.1) (P<0.001 for both comparisons). The rates of major bleeding were 0.5% in the placebo group, 0.2% in the 2.5-mg apixaban group, and 0.1% in the 5-mg apixaban group. The rates of clinically relevant nonmajor bleeding were 2.3% in the placebo group, 3.0% in the 2.5-mg apixaban group, and 4.2% in the 5-mg apixaban group. The rate of death from any cause was 1.7% in the placebo group, as compared with 0.8% in the 2.5-mg apixaban group and 0.5% in the 5-mg apixaban group.
Conclusions
Extended anticoagulation with apixaban at either a treatment dose (5 mg) or a thromboprophylactic dose (2.5 mg) reduced the risk of recurrent venous thromboembolism without increasing the rate of major bleeding.
