DPP-4 inhibitor did not increase adverse events in diabetic patients at high risk
An analysis of EXAMINE shows that in diabetic patients with a recent acute coronary syndrome, the combination of metformin, sulfonylurea and alogliptin was well tolerated.
Alogliptin in Patients with Type 2 Diabetes on Metformin and Sulfonylurea Therapies in the EXAMINE TrialLiterature - White WB, Heller SR, Cannon CP, et al. - Am J Med. 2018; published online ahead of print
Introduction and methods
The use of dipeptidyl dipeptidase-4 (DPP-4) inhibitors on top of metformin and sulfonylurea therapy is an effective and well tolerated combination, leading to significant improvements in glycated hemoglobin (HbA1c), but bearing an increased risk of hypoglycemia [1,2].
The Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) Trial was a large cardiovascular (CV) safety study in patients with type 2 diabetes (T2DM) and a recent acute coronary syndrome (ACS), who were randomized to receive alogliptin or placebo on top of standard-of-care-treatment. The study showed comparable CV event rates in the alogliptin and the placebo groups [3,4].
In the present analysis from the EXAMINE Trial, the anti-hyperglycemic efficacy and safety of the addition of alogliptin vs placebo was evaluated in a subgroup receiving metformin and sulfonylurea at baseline (N=1,398). Participants were followed for up to 40 (median 18) months. The change from baseline in HbA1c, adverse events, and CV outcomes were assessed.
Main results
- In patients receiving metformin and sulfonylurea only at baseline, the change in HbA1c was -0.4% ± 0.1% in the alogliptin group vs +0.1% ± 0.1% in the placebo group, with a least squares mean difference in change from baseline of -0.5% (95%CI: -0.7 to -0.4%; P <0.001).
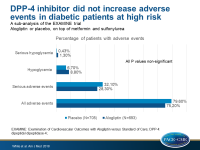
- For patients who were taking only metformin and sulfonylurea at baseline, the alogliptin and placebo groups did not differ in the proportion of patients with one or more adverse events (75.2% for alogliptin and 79.6% for placebo) or serious adverse events (28.3% for alogliptin and 32.1% for placebo).
- Although numerically increased, there was no significant difference in the incidence of hypoglycemia (8.8% for alogliptin and 6.7% for placebo; P=0.161) or serious hypoglycemia (1.30% for alogliptin and 0.43% placebo; P=0.088).
- Rates of CV death and all-cause mortality were significantly lower in patients randomized to alogliptin vs placebo (HR for CV death: 0.49; 95%CI: 0.28-0.84; P= 0.01; HR for all-cause mortality: 0.61; 95%CI: 0.38-0.96; P=0.033).
- There were no significant differences in non-fatal CV outcomes on alogliptin vs placebo, including hospitalization for heart failure or the combined endpoint of CV death or heart failure hospitalization.
Conclusion
In an analysis of the EXAMINE trial, in T2DM patients with a recent ACS, triple therapy with metformin, sulfonylurea and alogliptin led to significant reductions in HbA1c compared to dual therapy with metformin and sulfonylurea and was well tolerated. The observed reduction in CV death and all-cause mortality supports the CV safety of using alogliptin in a triple therapy regime in T2DM patients at high CV risk.
References
1. Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes. Diabetes Care 2013 36:3411–3417.
2. Scirica BM, Bhatt DL, Braunwald E, et al., SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326.
3. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327- 1335.
4. White WB, Bakris GL, Bergenstal RM, et al. Examination of cardiovascular outcomes with alogliptin versus standard of care in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J 2011; 162: 620-6.