First time randomized trial shows remission of T2DM with dietary and lifestyle intervention
The DIRECT trial evaluated a weight management program delivered by primary care. On average, weight reduced by 10 kg and almost half achieved and maintained remission of T2DM at 12 months after starting the intervention.
Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trialLiterature - Lean MEJ, Leslie WS, Barnes AC et al., - The Lancet, Available online 5 December 2017. https://doi.org/10.1016/S0140-6736(17)33102-1
See below to download our slide set about this study
Background
Current guidelines for management of type 2 diabetes (T2DM) focus on pharmacological treatment to reduce blood glucose and the associated elevated CV risk. Life expectancy is, however, still markedly reduced in T2DM patients.
The twin cycle hypothesis [1] postulates that T2DM is specifically caused by excess fat accumulation in the liver and pancreas. When the hypothesis was tested by means of inducing negative energy balance with a 600-700 kcal/day diet, liver insulin resistance and fat content normalized within 7 days, with first-phase insulin response and pancreas fat content normalizing over 8 weeks [2]. A subsequent parallel-group study showed that the underlying changes were stable over a 6-month period of isocaloric eating [3].
The current trial tested whether such an intervention is practical in routine primary care, as other studies that have shown weight loss of at least 10-15 kg and normalization of blood glucose in people with short-duration T2Dm, did not show sustained disease remission [4-7].
The Diabetes Remission Clinical Trial (DiRECT) [8] was an open-label, cluster-randomized trial at 49 primary care practices (unit of randomization) in participants diagnosed with T2DM within the previous 6 years, with BMI between 27 and 45 kg/m2. Current insulin use, HbA1c >12%, weight loss of >5 kg within the past 6 months, a recent eGFR<30 mL/min/1.73m2 and severe or unstable HF were among the exclusion criteria. After review of data from the first practices to enter the study, the diagnosis of T2DM were revised to specify that the most recent hbA1c value should be over 6.0%, and if it was <6.5% persons should still be receiving antidiabetic medication.
Participants in the intervention group were asked to follow the Counterweight-Plus weight management program [9] and aim for achieving and maintaining at least 15 kg weight loss. A total diet replacement phase with a low energy formula diet (825-853 kcal/day; 59% carbohydrate, 13% fat, 26% protein, 2% fiber) for 3 months was used, followed by structured food reintroduction of 2-8 weeks (about 50% carbohydrate, 25% total fat, 15% protein) and an ongoing structured program with monthly visits for long-term weight loss maintenance. All oral antidiabetic drugs were discontinued on day 1 of the program, with standard protocols for reintroduction according to national guidelines. Antihypertensive drugs were withdrawn in light of the rapid BP reductions seen upon commencement of a low energy diet. At the start of food reintroduction, physical activity strategies were introduced to help participants in the intervention group to reach and maintain their individual sustainable maximum.
Main results
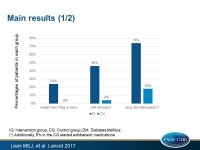
- Data for the first primary outcome of weight loss ≥15kg were available for 285 (96%) participants: n=137 intervention, n=148 control) and for the second primary outcome of remission of diabetes, defined as HbA1c <6·5% after at least 2 months off all antidiabetic medications, from baseline to month 12, were obtained for 290 (97%) persons (n=142 and n=148, respectively).
- At 12 months, weight loss of at least 15 kg was seen in 26 (24%) of participants in the intervention group and in no control participants.
- Diabetes remission was seen in 68 (46%) of intervention participants and in six (4%) of control participants (OR: 19.7, 95%CI: 7.8-49.8, P<0.0001).
- The intervention group showed on average 10.0 kg weight loss and the control group 1.0 kg (adj difference: -8.8, 95%CI: -10.3 to -7.3, P<0.0001). BMI and weight change as a percentage of baseline weight showed similar patterns.
- In participants following the intervention, weight fell sharply during the total diet replacement phase, by 14.5 kg on average (95%CI: 13.4-15.5) followed by a small increase during the food introduction phase (1.0 kg, 95%CI: 0.3-1.6) and the weight loss management phase (1.9 kg, 95%CI: 1.2-2.5).
- Mean HbA1c reduced by -0.9% (SD: 1.4) in the intervention group, and increased with 0.1% (SD: 1.1) in the control group (adj difference: -0.85%, 95%CI: -1.10 to -0.59, P<0.0001).
- At 12 months, 74% of 148 participants in the intervention group were taking no antidiabetic medication, compared with 18% of 148 controls, and in the latter group eight patients commenced antidiabetic medication.
- Quality of life improved by 7.2 points (SD: 21.3) on the EQ-5D visual analogue scale in the intervention group, while it decreased by 2.9 points (SD: 15.5) in the control group.
- While antihypertensive drugs had been withdrawn in the 38 (48%) intervention participants who took them at baseline, mean BP at 12 months was similar between groups. At 12 months, antihypertensive drugs were prescribed to 32% of participants in the intervention group and in 61% of the control group.
- Nine serious adverse events (7 in intervention, 2 in control group) were reported during the 12 months of follow-up. Two serious events (reported in the same individual), biliary colic and abdominal pain, were deemed potentially related to the intervention.
Conclusion
These results show that T2DM of up to 6 years’ duration can be reversed by weight loss with help of an evidence-based structured weight management program delivered in a community setting, by routine primary care staff. Almost a quarter of participants who followed the intervention achieved at least 15 kg of weight loss at 12 months, and half maintained at least 10 kg reduction. Almost half of patients in the intervention group showed remission of diabetes, and were off antidiabetic medication. Remission was closely related to the degree of weight loss maintained at 12 months. This cohort will be followed up for at least 4 years.
Editorial comment
To date, no findings from large-scale randomized trials were available on the effects of non-pharmacological treatment on remission of T2DM in patients receiving antidiabetic medication. Uusitupa [10] concludes that the obtained results are impressive and provide strong support for the view that T2DM is tightly associated with excessive fat mass in the body. These results, along with some other studies on T2DM prevention and some smaller intervention studies indicated that weight loss should be the primary goal in the treatment of T2DM, since it “results in improved insulin sensitivity in muscles and liver, decreases intra-organ fat content, and it might improve insulin secretion. In the long-term, weight loss might help to preserve β-cell mass. Once of the putative mechanisms could be decreased fat content of the pancreas, but more mechanistic studies are needed.” The role of physical activity and quality of diet, including dietary fibre and fatty acid composition are also important when considering the long-term success of prevention and treatment of T2DM.
The long-term results of the DiRECT study are important, because post-intervention weight regain is common among weight management studies in non-diabetic and diabetic populations. Uusitupa states “In view of the results of the DiRECT trial, a non-pharmacological approach should be revived. In clinical practice, anti- diabetic drugs seldom result in normalisation of glucose metabolism if patients’ lifestyles remain unchanged.”Uusitupa thinks that the time of diabetes diagnosis appears the best time point to start weight reduction and lifestyle changes, because the motivation of a patient is usually high.
References
1. Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia 2008; 51: 1781–89.
2. Lim EL, Hollingsworth KG, Aribisala BS et al. Reversal of type 2 diabetes: normalisation of beta cell
function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011; 54: 2506–14.
3. Steven S, Hollingsworth KG, Al-Mrabeh A, et al. Very Low calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care 2016; 39: 158–65.
4. Henry RR, Schaeffer L, Olefsky JM. Glycaemic effects of intensive caloric restriction and isocaloric refeeding in non-insulin dependent diabetes mellitus. J Clin Endocrinol Metab 1985; 61: 917–25.
5. Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014; 311: 2297–2304.
6. Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med 2013; 273: 219–34.
7. Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003; 238: 467–84.
8. Leslie WS, Ford I, Sattar N, et al. The Diabetes Remission Clinical Trial (DiRECT): protocol for a cluster randomised trial. BMC Fam Pract 2016; 17: 20.
9. Lean M, Brosnahan N, McLoone P, et al. Feasibility and indicative results from a 12-month low-energy liquid diet treatment and maintenance programme for severe obesity. Br J Gen Pract 2013;
63: e115–124.10]
10. Uusitupa M. Remission of type 2 diabetes: mission not impossible. The Lancet, Available online 5 December 2017