Liraglutide vs exenatide in type 2 diabetes
Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study.
Literature -Buse JB, Nauck M, Forst T, et al.
Lancet. 2013;381:117-24. doi: 10.1016/S0140-6736(12)61267-7.
In DURATION-6, 911 type 2 diabetes patients at 105 sites in 19 countries were randomized in an open-label parallel-group design to once-daily injections of liraglutide (1.8 mg; n=450) or once-weekly exenatide (2 mg; n=461), and results were analyzed on an intention-to-treat basis. The participants had already undergone lifestyle modification and were taking maximum or near-maximum doses of oral antihyperglycemic drugs but still had suboptimal glycemic control. The primary end point was change in HbA1c from baseline to six months.
Treatment with either exenatide once weekly or liraglutide once daily provided effective glucose lowering combined with weight loss and minimum hypoglycaemic episodes in patients with type 2 diabetes. The differences noted between the clinical efficacy of liraglutide once daily and exenatide once weekly at the doses tested, together with frequency and method of injection, can be used by clinicians in shared decision making for treatment of patients with type 2 diabetes uncontrolled by oral antihyperglycaemic drugs.
1. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–705.
2. Bergenstal RM, Wysham C, MacConell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010; 376: 431–39.
3. Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 2010; 375: 2234–43.
4. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372: 1240–50.
5. Blevins T, Pullman J, Malloy J, et al. DURATION-5: Exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96: 1301–10.
6. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human GLP-1 analog liraglutide in combination with metformin and TZD in patients with type 2 diabetes mellitus (LEAD-4 Met+TZD). Diabetes Care 2009; 32: 1224–30.
7. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–55.
8. Nauck M, Frid A, Hermansen K, et al. Eff cacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32: 84–90.
9. Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26: 268–78.
10. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374: 39–47.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373: 473–81.
12. Thethi T and Fonseca V. Comparing diabetes drugs—helping clinical decisions? Lancet 2013; 381:93-94
Background:
Glucagon-like peptide-1 receptor agonists exenatide and liraglutide have been shown to improve glycaemic control and reduce bodyweight in patients with type 2 diabetes. We compared the efficacy and safety of exenatide once weekly with liraglutide once daily in patients with type 2 diabetes.
Methods:
We did a 26 week, open-label, randomised, parallel-group study at 105 sites in 19 countries between Jan 11, 2010, and Jan 17, 2011. Patients aged 18 years or older with type 2 diabetes treated with lifestyle modification and oral antihyperglycaemic drugs were randomly assigned (1:1), via a computer-generated randomisation sequence with a voice response system, to receive injections of once-daily liraglutide (1•8 mg) or once-weekly exenatide (2 mg). Participants and investigators were not masked to treatment assignment. The primary endpoint was change in glycated haemoglobin (HbA(1c)) from baseline to week 26. Analysis was by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT01029886.
Findings:
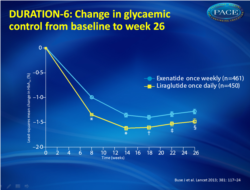
Of 912 randomised patients, 911 were included in the intention-to-treat analysis (450 liraglutide, 461 exenatide). The least-squares mean change in HbA(1c) was greater in patients in the liraglutide group (-1•48%, SE 0•05; n=386) than in those in the exenatide group (-1•28%, 0•05; 390) with the treatment difference (0•21%, 95% CI 0•08-0•33) not meeting predefined non-inferiority criteria (upper limit of CI <0•25%). The most common adverse events were nausea (93 [21%] in the liraglutide group vs 43 [9%] in the exenatide group), diarrhoea (59 [13%] vs 28 [6%]), and vomiting 48 [11%] vs 17 [4%]), which occurred less frequently in the exenatide group and with decreasing incidence over time in both groups. 24 (5%) patients allocated to liraglutide and 12 (3%) allocated to exenatide discontinued participation because of adverse events.
Interpretation:
Both once daily liraglutide and once weekly exenatide led to improvements in glycaemic control, with greater reductions noted with liraglutide. These findings, plus differences in injection frequency and tolerability, could inform th
Lancet. 2013;381:117-24. doi: 10.1016/S0140-6736(12)61267-7.
Background
GLP-1 receptor agonists are a fairly new class of antihyperglycemic drugs for patients with type 2 diabetes [1] and are licensed for use in addition to first-line agents such as metformin. Exenatide and liraglutide are examples of GLP-1 agonists and are approved for once-weekly (exenatide) or once-daily (liraglutide) use in patients with type 2 diabetes [2-11]. Use of these novel agents is limited at the moment, mostly because of their cost.In DURATION-6, 911 type 2 diabetes patients at 105 sites in 19 countries were randomized in an open-label parallel-group design to once-daily injections of liraglutide (1.8 mg; n=450) or once-weekly exenatide (2 mg; n=461), and results were analyzed on an intention-to-treat basis. The participants had already undergone lifestyle modification and were taking maximum or near-maximum doses of oral antihyperglycemic drugs but still had suboptimal glycemic control. The primary end point was change in HbA1c from baseline to six months.
Main results
- Both GLP-1 agonists led to improvements in glycemic control, with greater reductions noted with liraglutide: -1.48% compared with -1.28% for the exenatide group (fig.1).
- The overall difference in HbA1c between the two was small (0.21%)
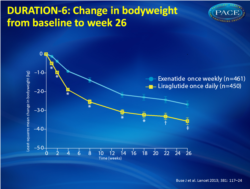
- Both treatments were associated with progressive decreases in body weight (fig. 2). Patients taking liraglutide lost more weight than those taking exenatide, irrespective of BMI.
- Gastrointestinal side effects—nausea, vomiting and diarrhea—were twice as common with liraglutide.
Conclusion
Treatment with either exenatide once weekly or liraglutide once daily provided effective glucose lowering combined with weight loss and minimum hypoglycaemic episodes in patients with type 2 diabetes. The differences noted between the clinical efficacy of liraglutide once daily and exenatide once weekly at the doses tested, together with frequency and method of injection, can be used by clinicians in shared decision making for treatment of patients with type 2 diabetes uncontrolled by oral antihyperglycaemic drugs.Editorial comment [12]
“There are some criticisms of the study duration and dose of liraglutide, among other things. However, the trial was well done, and even though it did not meet its primary end point, its results might help some clinicians to make appropriate treatment choices on the basis of relative efficacy and the risk of short-term side effects."
References
1. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–705.2. Bergenstal RM, Wysham C, MacConell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet 2010; 376: 431–39.
3. Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet 2010; 375: 2234–43.
4. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372: 1240–50.
5. Blevins T, Pullman J, Malloy J, et al. DURATION-5: Exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96: 1301–10.
6. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human GLP-1 analog liraglutide in combination with metformin and TZD in patients with type 2 diabetes mellitus (LEAD-4 Met+TZD). Diabetes Care 2009; 32: 1224–30.
7. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–55.
8. Nauck M, Frid A, Hermansen K, et al. Eff cacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32: 84–90.
9. Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26: 268–78.
10. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009; 374: 39–47.
11. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373: 473–81.
12. Thethi T and Fonseca V. Comparing diabetes drugs—helping clinical decisions? Lancet 2013; 381:93-94
Abstract
Background:
Glucagon-like peptide-1 receptor agonists exenatide and liraglutide have been shown to improve glycaemic control and reduce bodyweight in patients with type 2 diabetes. We compared the efficacy and safety of exenatide once weekly with liraglutide once daily in patients with type 2 diabetes.
Methods:
We did a 26 week, open-label, randomised, parallel-group study at 105 sites in 19 countries between Jan 11, 2010, and Jan 17, 2011. Patients aged 18 years or older with type 2 diabetes treated with lifestyle modification and oral antihyperglycaemic drugs were randomly assigned (1:1), via a computer-generated randomisation sequence with a voice response system, to receive injections of once-daily liraglutide (1•8 mg) or once-weekly exenatide (2 mg). Participants and investigators were not masked to treatment assignment. The primary endpoint was change in glycated haemoglobin (HbA(1c)) from baseline to week 26. Analysis was by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT01029886.
Findings:
Of 912 randomised patients, 911 were included in the intention-to-treat analysis (450 liraglutide, 461 exenatide). The least-squares mean change in HbA(1c) was greater in patients in the liraglutide group (-1•48%, SE 0•05; n=386) than in those in the exenatide group (-1•28%, 0•05; 390) with the treatment difference (0•21%, 95% CI 0•08-0•33) not meeting predefined non-inferiority criteria (upper limit of CI <0•25%). The most common adverse events were nausea (93 [21%] in the liraglutide group vs 43 [9%] in the exenatide group), diarrhoea (59 [13%] vs 28 [6%]), and vomiting 48 [11%] vs 17 [4%]), which occurred less frequently in the exenatide group and with decreasing incidence over time in both groups. 24 (5%) patients allocated to liraglutide and 12 (3%) allocated to exenatide discontinued participation because of adverse events.
Interpretation:
Both once daily liraglutide and once weekly exenatide led to improvements in glycaemic control, with greater reductions noted with liraglutide. These findings, plus differences in injection frequency and tolerability, could inform th

Facebook Comments