Mipomersen lowers LDL-C in statin-intolerant patients
03/05/2012
A randomized, double-blind, placebo-controlled phase 2 study was performed to evaluate the safety and efficacy of mipomersen in statin-intolerant patients with high CVD risk.
Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial.Literature - Visser ME, et al, Eur Heart J. 2012 Apr 16
Visser ME, Wagener G, Baker BF, Geary RS, Donovan JM, Beuers UH, Nederveen AJ, Verheij J, Trip MD, Basart DC, Kastelein JJ, Stroes ES.
Eur Heart J. 2012 Apr 16.
Background
Statins are widely used to lower LDL-C levels in patients at increased risk for cardiovascular disease [1]. In some patients, side effects lead to discontinuation of therapy, also due to the use of higher doses required to achieve LDL-C targets [1,2].Mipomersen is an antisense oligonucleotide inhibiting the synthesis of apolipoprotein B-100 [3], which is a main component of atherogenic lipid particles and is required for the secretion of VLDL from the liver. It has been shown to lower LDL-C and other apoB-containg lipoproteins.
A randomized, double-blind, placebo-controlled phase 2 study was performed to evaluate the safety and efficacy of mipomersen in statin-intolerant patients with high CVD risk. Patients with hypercholesterolaemia (n=33) were randomized to mipomersen (n=21) vs placebo (n=12). Mipomersen was administered at a dose of 200 mg weekly s.c. with matching placebo for 26 weeks, followed by a monitoring period of 6 months.
Main results
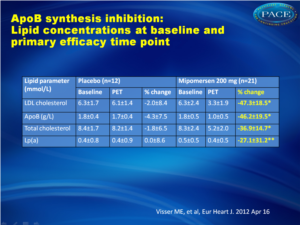
There was a 47% reduction (±18%, P < 0.001), in the primary efficacy endpoint of LDL 2 weeks after the last dose of mipomersen, compared with placebo, with a similar reduction of 46% (±20%, P < 0.001) in mean apoB levels. There was a differential effect on LDL particles, with maximal lowering of the small particles as compared with the larger ones. Additional lipid parameters were also affected by mipomersen, with a reduction in triglycerides of 27% and an increase in HDL of 8%. Adverse effects included injection site reactions in 95% of the patients in the mipomersen arm and 83% in the placebo arm, although no patients discontinued study treatment due to injection site reactions. Only six patients discontinued treatment prematurely due to side effects (4 in the mipomersen arm, 2 in the placebo arm), resulting in a >80% compliance rate. Increases in ALT above the ULN were more common in the mipomersen treatment group [n = 17 (81%)] compared with the placebo treatment group [n = 3 (25%)]. Persistent increases in ALT (≥3× ULN on two consecutive occasions at least 7 days apart) were observed in seven subjects (33%) from the active treatment group. After discontinuation of treatment, transaminases returned to normal. Hepatic steatosis was observed in a substantial proportion of mipomersen-treated subjects with ALT ≥ 2x ULN.Conclusion
Mipomersen is a promising treatment option for patients at high risk for CVD who are statin-intolerant. Long-term safety data have to be awaited.
Editorial comment
“These results provide evidence on the efficacy and safety of mipomersen in lowering LDL-cholesterol and apoB in statin-intolerant patients. Mipomersen offers an intriguing alternative. However, there is a highly significant incidence of adverse effects related both to the administration (injection site reactions) and the mechanism (IHTG accumulation, LFT abnormalities) of the drug that must be noted. Therefore, when choosing mipomersen therapy, extra vigilance will be needed to monitor the patient closely for LFT abnormalities and fatty acid accumulation in the liver. Since these adverse effects appear to be duration and dose dependent, intermittent dosing or lower doses of mipomersen may also be a strategic option in statin-intolerant patients who might be at higher risk for these adverse effects. Injection site reactions may limit the maximally tolerated dosing interval. In the statin era in which we currently practice, mipomersen will never become first-line therapy for hyperlipidaemia, but, given its efficacy, it holds promise as a second- or third-line agent for the particularly challenging high-risk patients who cannot tolerate statins to achieve their LDL targets.
References
1. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment III) final report. Circulation 2002;106:3143–3421.2. Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12 064 survivors of myocardial infarction: a double-blind randomized trial. Lancet 2010;376:1658–1669.
3. Crooke RM, Baker BF, Wedel M. Cardiovascular Therapeutic Applications in Antisense Drug Technology; Principles, Strategies and Applications, 2nd ed. Boca Raton, FL: CRC Press; 2007. p. 601–639.
Abstract
AimsA randomized, double-blind, placebo-controlled study was conducted to investigate the safety and efficacy of mipomersen, an apolipoprotein B-100 (apoB) synthesis inhibitor, in patients who are statin intolerant and at high risk for cardiovascular disease (CVD).
Methods and results
Thirty-three subjects, not receiving statin therapy because of statin intolerance, received a weekly subcutaneous dose of 200 mg mipomersen or placebo (2:1 randomization) for 26 weeks. The primary endpoint was per cent change in LDL cholesterol (LDL-c) from the baseline to Week 28. The other efficacy endpoints were per cent change in apoB and lipoprotein a [Lp(a)]. Safety was determined using the incidence of treatment-emergent adverse events (AEs) and clinical laboratory evaluations. After 26 weeks of mipomersen administration, LDL-c was reduced by 47 ± 18% (P < 0.001 vs. placebo). apoB and Lp(a) were also significantly reduced by 46 and 27%, respectively (P < 0.001 vs. placebo). Four mipomersen (19%) and two placebo subjects (17%) discontinued dosing prematurely due to AEs. Persistent liver transaminase increases ≥3× the upper limit of normal were observed in seven (33%) subjects assigned to mipomersen. In selected subjects, liver fat content was assessed, during and after treatment, using magnetic resonance spectroscopy. Liver fat content in these patients ranged from 0.8 to 47.3%. Liver needle biopsy was performed in two of these subjects, confirming hepatic steatosis with minimal inflammation or fibrosis.
Conclusion
The present data suggest that mipomersen is a potential therapeutic option in statin-intolerant patients at high risk for CVD. The long-term follow-up of liver safety is required.