PPAR-γ agonist reduces stroke and MI in patients with insulin resistance after ischaemic event
Treatment with pioglitazone to improve insulin sensitivity, reduced stroke and MI in patients with insulin resistance but without diabetes and who have experienced ischaemic stroke or TIA.
Pioglitazone after Ischemic Stroke or Transient Ischemic AttackLiterature - Kernan WN et al., NEJM 2016
Kernan WN, Viscoli CM, Furie KL et al.
February 17, 2016DOI: 10.1056/NEJMoa1506930
Background
Patients who have experienced ischaemic stroke or transient ischaemic attack (TIA) are at increased risk for future CV events [1,2]. Insulin resistance increases the risk of vascular disease. Insulin resistance is not only seen in almost all patients with type 2 diabetes, but also in over 50% of patients without diabetes, who have had an ischemic stroke or TIA [3]. Treatment of insulin resistance may represent a potential new preventive strategy after ischemic stroke or TIA [4].Insulin sensitivity may be improved by exercise, diet, weight reduction and medications. The PPAR-γ agonist pioglitazone is approved as a glucose-lowering agent in patients with type 2 diabetes, and may reduce the risk of CV events including stroke [5,6].
The placebo-controlled Insulin Resistance Intervention after Stroke (IRIS) trial was set-up to evaluate whether pioglitazone reduces the rates of stroke and myocardial infarction (MI) after ischaemic stroke or TIA (within 6 months before randomisation) in patients without diabetes, with insulin resistance. Initial dose of pioglitazone was 15 mg, which was increased to two pills daily (30 mg) at 4 weeks and to 45 mg at 8 weeks, if patients reported no new or worsening oedema, shortness of breath, myalgia or excessive weight gain. 3876 patients were included (mean age 63.5 years). Median follow-up was 4.8 years.
Main results
- 175 out of 1939 patients in the pioglitazone group (9.0%) experienced the primary outcome of stroke or MI, as compared with 228 out 1937 (11.8%) in the placebo group (HR: 0.76, 95%CI: 0.62-0.93, P=0.007).
- Rate of progression to diabetes was lower in patients on pioglitazone than on placebo (HR: 0.48, 95%CI: 0.33-0.69, P<0.001).
- There was no difference in mean score on the Modified Mini-Mental State Examination between treatments (-0.02 with pioglitazone vs. placebo, 95%CI: -0.33 to 0.28, P=0.88).
- After 1 year, HOMA-IR index (4.1 vs. 5.7, P<0.0001)) and C-reactive protein level (3.3 vs. 4.4 mg/L, P=0.02) were lower after pioglitazone use than after placebo use.
- Patients on pioglitazone showed more weight gain, oedema (35.6% vs. 24.9%, P<0.001), shortness of breath than patients in the placebo group. Maximum between-group difference in weight change was seen after 4 years (mean 2.6 kg gain vs. mean 0.5 kg loss in placebo group, P<0.001). Serious bone fractures were seen in 5.1% of patients in the pioglitazone group, as compared with 3.2% of patients on placebo (P=0.003). Occurrence of or hospitalisation for heart failure did not differ between treatment arms.
Download Kernan NEJM 2016_IRIS_PACE.pptx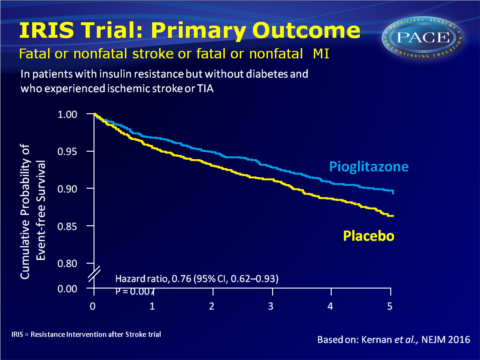
Conclusion
Treatment with PPAR-γ agonist pioglitazone in patients without diabetes and a recent history of ischemic stroke or TIA and with insulin resistance reduced the rate of stroke and MI as compared to placebo. Progression to diabetes also occurred less often with pioglitazone.Previously recognised adverse effects were now also observed, including weight gain. This reflects an increase in adipose tissue mass and a tendency for fluid accumulation. The safety algorithm included dose reduction in case of excessive weight gain or oedema, and pioglitazone treatment was not associated with more heart failure.
Find this article online at NEJM
References
1. Dhamoon MS, Sciacca RR, Rundek T, Et al. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology 2006; 66: 641-6.
2. Johnston SC. Transient ischemic attack. N Engl J Med 2002; 347: 1687-92.
3. Kernan WN, Inzucchi SE, Viscoli CM, et al. Impaired insulin sensitivity among nondiabetic patients with a recent TIA or ischemic stroke. Neurology 2003; 60: 1447-51.
4. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American
Heart Association/American Stroke Association. Stroke 2014; 45: 2160-236.
5. Wilcox R, Bousser M-G, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke 2007; 38: 865-73.
6. Dormandy JA, Charbonnel B, Eckland DJA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366: 1279-89.
