WOEST: aspirin not needed after PCI in patients treated with clopidogrel
Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial.
Literature - Dewilde WJ et al for the WOEST study investigators. - Lancet. 2013 Feb 12. doi:pii: S0140-6736(12)62177-1. 10.1016/S0140-6736(12)62177-1. [Epub]Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, Tijsen JG, van 't Hof AW, Ten Berg JM; for the WOEST study investigators.
Lancet. 2013 Feb 12. doi:pii: S0140-6736(12)62177-1. 10.1016/S0140-6736(12)62177-1. [Epub]
Background
Long-term treatment with oral anticoagulants is necessary in patients with mechanical heart valves and in most with atrial fibrillation [1-3]. Patients with an implanted coronary stent also need platelet inhibition with aspirin and clopidogrel to prevent the rare but fatal complication of stent thrombosis [3,4]. Triple therapy with an oral anticoagulant, aspirin and clopidogrel, however, increases the risk of major bleeding and associated mortality [5-8].The What is the Optimal antiplatElet and anticoagulant therapy in patients with oral anticoagulation and coronary StenTing (WOEST) study is an investigator-driven study designed to answer a specific dilemma in cardiology: the optimal antithrombotic management of patients receiving oral anticoagulants (for atrial fibrillation or a mechanical valve) who undergo coronary stenting. The study is the first to study the safety of omitting of aspirin in this kind of patients.
The study randomized 573 patients to OAC (acenocoumarol or phenprocoumon) plus clopidogrel alone (double therapy) or OAC plus clopidogrel plus aspirin (triple therapy). Follow-up was one year, the primary endpoint was the occurrence of any bleeding (TIMI criteria), secondary endpoints were a combination of stroke, death, MI, stent thrombosis and target vessel revascularization, and also all the individual components of primary and secondary endpoints.
Main results
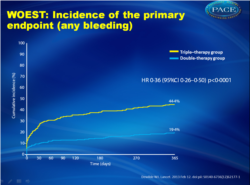
- After 1 year follow-up, bleeding occurred in 54 (19.4%) patients in the dual therapy group and in 126 patients (44.4%) in the triple therapy group (Figure 1). In the dual therapy group six (2.2%) patients had multiple hemorrhages, compared with 34 (12.0%) in the triple therapy group.
- After 1 year at least one blood transfusion was needed in 11 (3.9%) patients on dual therapy, compared with 27 (9.5%) of patients on triple therapy (odds ratio of Kaplan-Meier curve 0.39 , 95% CI 0.17 to 0.84, p = 0.011).
- The combined secondary endpoint of death, myocardial infarction, stroke, target vessel revascularization and stent thrombosis was reported in 31 (11.1%) patients in the dual therapy group and in 50 (17.6%) in the triple therapy group (Fig. 2 ). After correction for imbalance in baseline characteristics the HR remained similar (0.56, 95% CI 0.35 -0.91). Seven patients (2.5%) in the dual therapy group and 18 (6.3%) in the triple therapy group died from all causes after 1 year.
Conclusion
Use of clopidogrel without aspirin leads to less bleeding, without increasing the risk of thrombotic events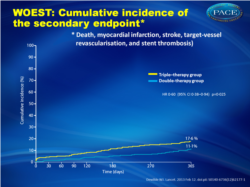 Cumulative incidence of the secondary endpoint (death, myocardial infarction, stroke, target-vessel Cumulative incidence of the secondary endpoint (death, myocardial infarction, stroke, target-vesselrevascularisation, and stent thrombosis) |
References
1. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114: e84–231.2. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31: 2369–429.
3. Lip GY, Huber K, Andreotti F, et al. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary intervention/ stenting. Thromb Haemost 2010; 103: 13–28.
4. Smith SC Jr, Feldman TE, Hirshfeld JW Jr, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). Circulation 2006; 113: 156–75.
5. Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med 2010; 170: 1433–41.
6. Karjalainen PP, Porela P, Ylitalo A, et al. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J 2007; 28: 726–32.
7. Orford JL, Fasseas P, Melby S, et al. Safety and efficacy of aspirin, clopidogrel, and warfarin after coronary stent placement in patient with an indication for anticoagulation. Am Heart J 2004; 147: 463–67.
8. Doyle BJ, Rihal CS, Gastineau DA, Holmes DR Jr. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009; 53: 2019–27.
Abstract
Background:If percutaneous coronary intervention (PCI) is required in patients taking oral anticoagulants, antiplatelet therapy with aspirin and clopidogrel is indicated, but such triple therapy increases the risk of serious bleeding. We investigated the safety and efficacy of clopidogrel alone compared with clopidogrel plus aspirin.
Methods:
We did an open-label, multicentre, randomised, controlled trial in 15 centres in Belgium and the Netherlands. From November, 2008, to November, 2011, adults receiving oral anticoagulants and undergoing PCI were assigned clopidogrel alone (double therapy) or clopidogrel plus aspirin (triple therapy). The primary outcome was any bleeding episode within 1 year of PCI, assessed by intention to treat. This study is registered with ClinicalTrials.gov, number NCT00769938.
Findings:
573 patients were enrolled and 1-year data were available for 279 (98•2%) patients assigned double therapy and 284 (98•3%) assigned triple therapy. Mean ages were 70•3 (SD 7•0) years and 69•5 (8•0) years, respectively. Bleeding episodes were seen in 54 (19•4%) patients receiving double therapy and in 126 (44•4%) receiving triple therapy (hazard ratio [HR] 0•36, 95% CI 0•26-0•50, p<0•0001). In the double-therapy group, six (2•2%) patients had multiple bleeding events, compared with 34 (12•0%) in the triple-therapy group. 11 (3•9%) patients receiving double therapy required at least one blood transfusion, compared with 27 (9•5%) patients in the triple-therapy group (odds ratio from Kaplan-Meier curve 0•39, 95% CI 0•17-0•84, p=0•011).
Interpretation:
Use of clopiogrel without aspirin was associated with a significant reduction in bleeding complications and no increase in the rate of thrombotic events.

Facebook Comments