PCSK9-inhibition safe and effective in heterozygous and homozygous familial hypercholesterolaemia
The PCSK9-antibody evolocumab lowered LDL-c in patients with HoFH and HeFH, as seen in the TESLA part B and RUTHERFORD-2 studies. Mutation information can help to assess treatment response in HoFH, but not in HeFH.
Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial.Literature - Raal et al., The Lancet 2014
Raal FJ, Honarpour N, Blom DJ et al.
PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial.
Raal FJ, Stein EA, Dufour R et al.Lancet. 2014 Oct 1. doi: 10.1016/S0140-6736(14)61399-4. [Epub ahead of print]
Background
Patients with homozygous familial hypercholesterolaemia (HoFH) show very low plasma LDL clearance, substantially raised LDL-c and early cardiovascular morbidity and mortality. HoFH is the consequence of mutations in either the LDL-receptor alleles (over 95% of patients), or those of apolipoprotein B, or PCSK9 or the LDL-receptor adaptor protein [1,2].Heterozygous FH (HeFH) can be the result of mutations in the same genes, although in up to 20% of patients with a clinical diagnosis, no mutation can be identified [3]. HeFH is estimated to affect between one in 250 and one in 300 people worldwide [4,5]. Residual LDL receptor activity is associated with the severity of LDL-c increase and propensity for early CV disease [6,7].
Conventional therapy with statins and ezetimibe can lower LDL-c levels by about 40-45% on average [8-10]. Despite the recent approval of mipomersen (inhibiting apolipoprotein B synthesis) by the FDA and the EMA and of lomitapide (inhibiting microsomal triglyceride transfer protein) by the EMA for the treatment of HoFH, LDL-c levels remain substantially high in nearly all patients with HoFH, as well as in patients with HeFH.
Evolocumab is a fully human monoclonal antibody that binds to PCSK9, which targets the LDL-receptor for degradation. Evolocumab allows increased recycling of LDL-receptors and lowers LDL-c levels by 60% in patients with heFH [11].
The Trial Evaluating PCSK9 Antibody in Subjects with LDL Receptor Abnormalities (TESLA) part B study was a randomised, double-blind, placebo-controlled phase 3 trial testing the efficacy and safety of evolocumab in patients with HoFH. RUTHERFORD-2 was a multicentre, randomised, double-blind, placebo-controlled study to assess safety and efficacy of evolocumab administered biweekly or every month in a large and diverse cohort of patients with HeFH with LDL-c >2.6 mmol/L despite intense lipid-lowering therapy. TESLA and RUTHERFORD-2 were published simultaneously in The Lancet.
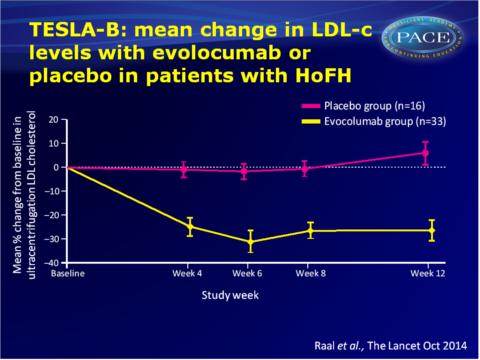
Main results TESLA part B
- Least-squares mean percentage reduction in LDL-c from baseline after 12 weeks of treatment with evolocumab was 23.1% (95%CI: -30.7 to -15.4), which was 30.9% lower (95%CI: -43.9 to -18.0) than with placebo (adjusted P<0.0001). Mean least-squares absolute reduction with evolocumab vs. placebo was 2.4 mol/L (95%CI: -3.7 to -1.1).
- In the 7 adolescent patients (<18 years) receiving evolocumab the least-squares mean reduction in LDL-c from baseline at week 12 was 26.0% (95%CI: -49.9 to -2.2).
- Depending on how many LDL-receptor alleles were defective, a different average response to treatment was seen.
- Even among genetically confirmed HoFH patients with the same LDL-receptor mutations variable responses to evolocumab were seen.
- Treatment with evolocumab also significantly decreased mean apolipoprotein B levels at week 12 vs. placebo, but not lipoprotein (a) levels.
- No serious adverse events were reported, and more treatment-emergent adverse events occurred in the placebo (62%) group than in the evolocumab (36%) group.
Download Raal Lancet TESLA 2014 PACE.pptx
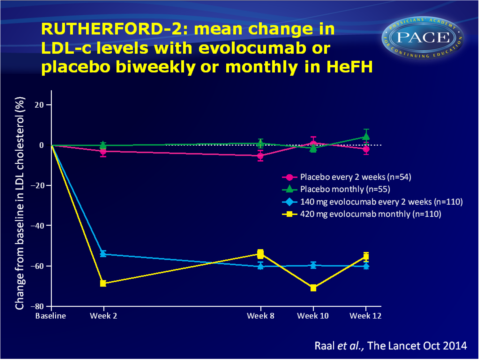
Main results RUTHERFORD-2
- As compared with placebo, biweekly evolocumab 140 mg yielded mean LDL-c reductions of 59.2% (95%CI: 53.4-65.1) at week 12. Monthly administration of evolocumab 420 mg resulted in mean reductions at week 12 of 61.3% (95%CI: 53.6-69.0) vs. placebo.
- At week 12, LDL-c<1.8 mmol/L was achieved by 71 out of 104 (68%) patients on evolocumab 140 mg Q2W and by 65 out of 103 (63%) patients on evolocumab 420 mg monthly, as compared with one patient in each placebo group.
- Mean reductions in lipoprotein(a) and apolipoprotein B at week 12 were significantly greater in both evolocumab groups as compared with the placebo groups. Triglyceride levels were also significantly lower with evolocumab, albeit to a larger extent with biweekly 140 mg. HDL-c was significantly increased with evolocumab as compared with placebo.
- LDL-c reduction was comparable between patients with receptor-negative or receptor-defective activity or in patients with unclassified LDL-receptor status. Also patients who were genetic homozygotes or compound heterozygotes showed similarly large reductions with evolocumab.
- Similar rates of adverse events were reported in the evolocumab and placebo groups, and no serious adverse events led to discontinuation of the study drug, nor were judged to be related to the study drug.
Download Raal Lancet Rutherford 2014 pace.pptx
Conclusions
The TESLA part B study confirms data from the pilot trial that PCKS9 inhibition with monthly subcutaneous evolocumab 420 mg lowers LDL-c by 30.9% as compared to placebo. This study shows that genetic information gives insight into possible response to treatment of HoFH, and genetic confirmation of HoFH could be used to select patients for evolocumab treatment. In most HoFH patients, with at least one defective LDL-receptor mutation, LDL-c reduction was 41%, thus many patients in this difficult-to-treat population appear to benefit from evolocumab.The RUTHERFORD-2 study shows that addition of evolocumab treatment to existing lipid-lowering therapy gives LDL-c reductions of about 60%, with a majority of patients achieving LDL-c<1.8 mmol/L. Patients with receptor-negative mutations responded equally well to treatment as those with defective mutations, suggesting that the response to PCSK9 inhibition with evolocumab in HeFH depends mostly on upregulation of the non-affected LDL-receptor, while the mutant receptor plays little role. Thus, in patients with HeFH genetic analysis might not be helpful in assessment of response to evolocumab.
The variability of the response to evolocumab within patient groups with different genetic characteristics as seen in both studies suggests that other factors than the receptor mutation alone must also play a role in LDL-c reduction with evolocumab.
Editorial comment [12]
“Despite the short duration of these studies, their findings are compatible with longer-term observations in other groups of patients with hypercholesterolaemia and they provide good-quality data that will advance the future care of familial hypercholesterolaemia. Molecular testing, orassessment of residual LDL receptor function in fibroblasts, might be useful to identify patients with homozygous familial hypercholesterolaemia who are likely to respond to PCSK9 inhibitors. However, this approach would not apply to patients with the heterozygous form of the disorder. If proven to be safe and efficacious in the long term, as well as cost effective, PCSK9 monoclonal antibodies might be
the best standard of care for many patients with severe forms of familial hypercholesterolaemia.”
Find these articles on Pubmed
TESLA Part B. Raal et al., The Lancet Oct 1 2014
RUTHERFORD-2. Raal et al., The Lancet Oct 1 2014
Editorial comment. Santos RD and Watts GF. The Lancet Oct 1 2014
References
1 Usifo E, Leigh SE, Whittall RA, et al. Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Ann Hum Genet 2012; 76: 387–401.
2 Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J 2014; published online Feb 28. DOI:10.1093/eurheartj/ehu058
3 Vandrovcova J, Thomas ERA, Atanur SS, et al. The use of next-generation sequencing in clinical diagnosis of familial hypercholesterolemia. Genet Med 2013; 15: 948–57. 11 Neil A, Cooper J, Betteridge J, et al. Reductions in all
4 Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, et al, eds. The metabolic and molecular bases of inherited disease, 8th edn. New York: McGraw Hill, 2001: 2863–913.
5 Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013; 34: 3478–90a.
6 Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014; published online July 22. DOI:10.1093/eurheartj/ehu274.
7 Raal FJ, Santos RD. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis 2012; 223: 262–68.
8 Marais AD, Raal FJ, Stein EA, et al. A dose-titration and comparative study of rosuvastatin and atorvastatin in patients with homozygous familial hypercholesterolaemia. Atherosclerosis 2008; 197: 400–06.
9 Raal FJ, Pilcher GJ, Illingworth DR, et al. Expanded-dose simvastatin is effective in homozygous familial hypercholesterolaemia. Atherosclerosis 1997; 135: 249–56.
10 Gagne C, Gaudet D, Bruckert E, and the Ezetimibe Study group. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation 2002; 105: 2469–75.
11 Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 2012; 126: 2408–17.
12. Santos RD, Watts GF. Familial hypercholesterolaemia: PCSK9 inhibitors are coming. The Lancet. October 2, 2014 http://dx.doi.org/10.1016/S0140-6736(14)61702-5
