New fully human PCSK9 antibody successful in phase 2 trial
RG7652, a PCSK9 antibody, dose-dependently reduced LDL-c, ApoB and Lp(a), but not inflammatory proteins, without concerning adverse events.
Effects of RG7652, a Monoclonal Antibody Against PCSK9, on LDL-C, LDL-C Subfractions, and inflammatory Biomarkers in Patients at High Risk of or With Established Coronary Heart DiseaseLiterature - Baruch A, Mosesova S, Davis JD, et al. - Am J Cardiol. 2017 Mar 1, Epub ahead of print
Background
Atherosclerosis has, additional to the role of LDL-c, a strong inflammatory component that can affect vascular health and plaque stability [1-4]. Therefore, this multicenter, randomized, double-blind, placebo-controlled phase 2 study, called EQUATOR, assessed the LDL-c lowering and the impact on inflammatory biomarkers by RG7652, a fully human PCSK9 antibody, when added to standard-of-care therapy for 6 months, in patients with a high risk for, or with established coronary heart disease (CHD). Patients were randomized to placebo (n=57 completed), RG7652 200 mg subcutaneously every 8 weeks (200 q8w, n=23 completed), 400 mg every 4 (400 q4w, n=52 completed) or 8 weeks (400 q8w, n=28 completed), 800 mg every 8 (800 q8w, n=46 completed) or 12 weeks (800 q12w, n=23 completed).
Main results
- Of the 248 patients randomized, 7.7% discontinued the study.
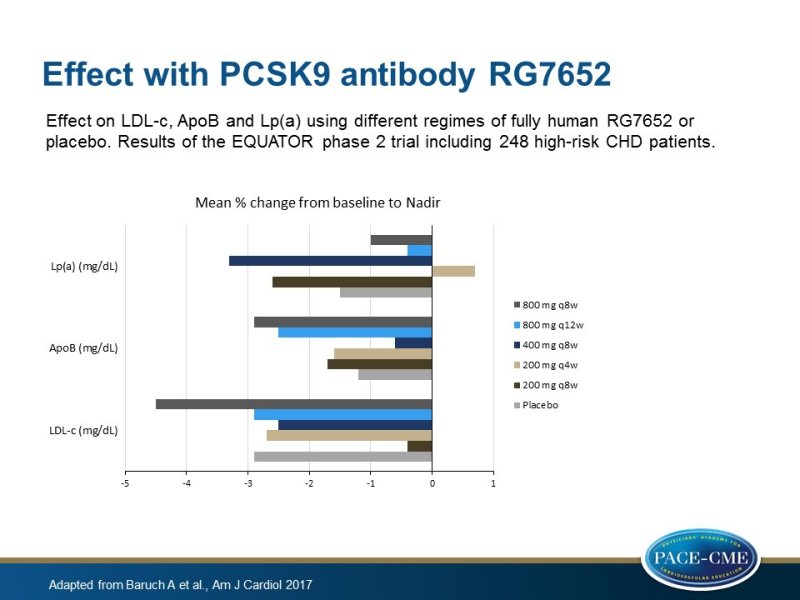
- Mean LDL-c at baseline was 125.8 mg/dL and the proportion of patients with baseline LDL-c <120 and ≥120 mg/dL were comparable in each group.
- Mean reductions LDL-c at day 167 were -7.7%, -10.7% (P=0.54), -58.7% (P<0.001), -23.3% (P=0.0013), -16.1% (P=0.029) and -44.3% (P<0.001) for placebo, 200 mg q8w, 200 mg q4w, 400 mg q8w, 800 mg q12w and 800 mg q8w, respectively. P values were compared to placebo.
- Results were similar between statin-treated and statin-intolerant patients and between diabetic and nondiabetic patients.
- Levels of ApoB were reduced with RG7652 with -5%, -5.3% (P=0.96), -46.7% (P<0.001), -16.6% (P=0.0023), -13.3% (P=0.038) and -35.6% (P<0.001), respectively. P values were compared to placebo.
- Levels of Lp(a) were changed with 4.6%, -3.7% (P=0.38), -25.5% (P<0.001), -14.9% (P=0.0058), -8.8% (P=0.034) and -22.5% (P<0.001), respectively. P values were compared to placebo.
- Characterization of lipoprotein variables focused on the 400 q4w and 800 q8w groups only. Both large and small LDL-c particles were significantly reduced in both groups on day 15, 85 and 169. Throughout treatment, large LDL-c was more reduced than small LDL-c (from baseline to day 169, -38% and -26% for small LDL in 400 q4w and 800 q8w respectively and -63% and -44% for large LDL in 400 q4w and 800 q8w respectively).
- Levels or inflammatory markers hsCRP or effector cytokines IL-6 and TNF-α were not significantly changed by RG7652.
- Adverse events (AEs) were observed in 86.3% of patients of all RG7652 treatment arms combined and in 87.5% of placebo patients. No apparent dose-related trend in AEs was observed. Most AEs were mild or moderately and the incidence of severe AEs were 9.3% and 10.9% in combined RG7652 arms and placebo arm respectively.
- Treatment-related AEs were more often experienced in RG7652-treated patients (34.4%) compared to placebo (25%).
- Cardiac AEs were noticed in 8.2% of RG7652 patients (4.4% serious) and in 7.8% of placebo patients (6.3% serious).
- Hypertension was more often experienced in RG7652-treated patients.
Conclusion
RG7652 significantly reduced LDL-c levels as well as ApoB and Lp(a) in high-risk CHD patients. The magnitude and duration of LDL-c-lowering were dose-dependent. On the other hand, RG7652 did not lower inflammatory markers and cytokines, suggesting that PCSK9 inhibition does not have the same effect on systemic inflammation as statins. Furthermore, no concerning AEs were experienced.
References
1. Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med 2012;367:1891e1900.
2. Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, Du Y, Kranz T, Gasparino E, Swergold GD. Effect of a monoclonal antibody to PCSK9 on LDL
cholesterol. N Engl J Med 2012;366:1108e1118.
3. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA. A 52-week
placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014;370:1809e1819.
4. 8. Ascer E, Bertolami MC, Venturinelli ML, Buccheri V, Souza J, Nicolau JC, Ramires JA, Serrano CV Jr. Atorvastatin reduces proinflammatory markers in hypercholesterolemic patients. Atherosclerosis
2004;177:161e166.