Bempedoic acid/ezetimibe combination plus atorvastatin robustly reduced LDL in phase 2
Targeting complementary mechanisms of action with bempedoic acid and ezetimibe on top of a statin in hypercholesterolemia, robustly reduced LDL-c levels as well as hsCRP, was safe and well tolerated in a 6-week phase 2 trial.
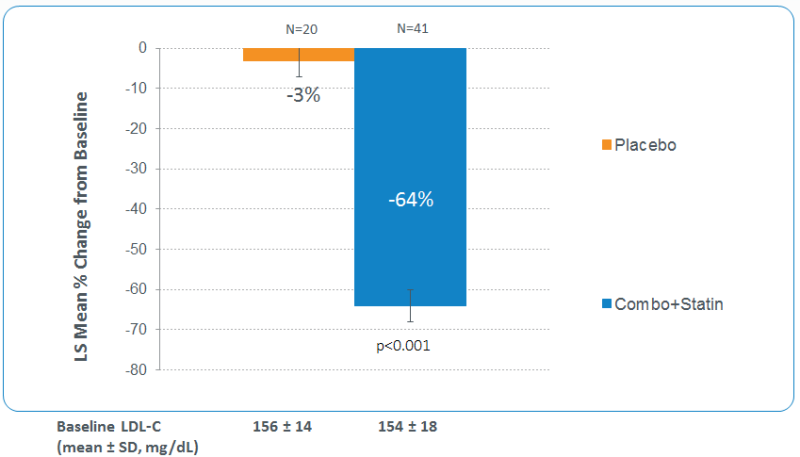
News - Aug. 15, 2017During a 6-week phase 2 trial (1002-038), also known as the triplet oral therapy study, the bempedoic acid/ezetimibe combination plus atorvastatin met its primary endpoint of >64% LDL-c-lowering from baseline, when compared to placebo (P<0.001) in patients with hypercholesterolemia. Ninety five percent of patients receiving treatment achieved greater than or equal to 50 percent LDL-c-lowering reduction and 90 percent achieved LDL-c levels of less than 70 mg/dL.
The bempedoic acid/ezetimibe combination plus atorvastatin also demonstrated a reduction of 48 percent (p<0.001) in high-sensitivity C-reactive protein (hsCRP), an important marker of the underlying inflammation associated with cardiovascular disease.
There were no reported serious adverse events (SAEs), no difference in muscle-related adverse events (AEs), or discontinuations due to muscle-related AEs, in the treatment group, as compared to the placebo group. The bempedoic acid/ezetimibe combination plus atorvastatin produced no elevations in liver function tests (ALT/AST) or creatine kinase (CK). The bempedoic acid/ezetimibe combination plus atorvastatin was observed to be safe and well-tolerated.
The 6-week, phase 2, randomized, double-blind, placebo-controlled study evaluated the efficacy and safety of bempedoic acid 180 mg, ezetimibe 10 mg and atorvastatin 20 mg versus placebo in patients with hypercholesterolemia. Secondary objectives include assessing the safety and tolerability of the bempedoic acid/ezetimibe combination plus atorvastatin therapy versus placebo, and effects on other risk markers, including hsCRP, non-high-density lipoprotein cholesterol (non-HDL-C), total cholesterol and apolipoprotein B (apoB). A total of 63 patients with hypercholesterolemia were washed out of any lipid-regulating therapies. Forty-three patients received the bempedoic acid/ezetimibe combination plus atorvastatin; 20 patients received placebo.
Bempedoic acid and ezetimibe have complementary mechanisms of action of inhibition of cholesterol synthesis (bempedoic acid) and inhibition of cholesterol absorption (ezetimibe). Inhibition of ATP Citrate Lyase (ACL) by bempedoic acid reduces cholesterol biosynthesis and lowers LDL-c by up-regulating the LDL receptor. Inhibition of Niemann-Pick C1-Like 1 (NPC1L1) by ezetimibe results in reduced absorption of cholesterol from the gastrointestinal tract, thereby reducing delivery of cholesterol to the liver, which in turn upregulates LDL receptors.