CANTOS results show that inhibiting inflammation reduces CV events and possibly lung cancer rates
ESC 2017 Inhibition of IL-1β with canakinumab significantly lowered hsCRP levels and CV events, especially in those with higher than median CRP reduction, and incident lung cancer and lung cancer mortality were reduced.
ESC 2017 - BarcelonaNews - Aug. 27, 2017
The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study
Presented at the ESC congress 2017 by: Paul M RIDKER (Boston, MA, USA)
Background
Over the past 25 years, many research efforts have been dedicated to evaluating how lowering LDL-c can lower CV events, a concept that originated in the 4S trial results. In parallel, researchers developed an interest in the effect of lowering inflammation in relation to CV risk. It was noted that a substantial proportion of myocardial infarctions (MI) occurs in people without elevated LDL. The JUPITER trial indeed showed a role for high C-reactive protein (CRP) in CV risk, but this trial did not address the question whether reducing inflammation could lower CV event risk.
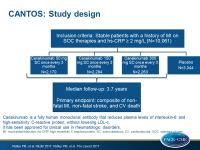
The randomized double-blind placebo-controlled CANTOS trial aimed to test whether reducing inflammation in 10061 patients (in 39 countries) with stable coronary artery disease (CAD, with prior MI) can lower the risk of another CV event. Patients were on statin therapy, ACE inhibition or ARB, betablockers, aspirin, and had persistently elevated hsCRP (≥ 2mg/L). Patients were randomized to canakinumab (50 mg or 150 mg or 300 mg subcutaneously (SC) once every 3 months) or placebo (SC once every 3 months), a human monoclonal antibody that neutralizes interleukin-1β (IL-1β) signaling. IL-1β is part of the NLRP3 inflammasome, which is part of the innate immune system. IL-1β activates IL-6, which in turn results in production of CRP, PAI-1 and fibrinogen in the liver.
The primary CV endpoint was non-fatal MI, non-fatal stroke, CV death (MACE). 1490 primary events were seen. A key secondary CV endpoint (MACE+) consisted of MACE plus unstable angina requiring unplanned revascularization. The critical non-CV safety endpoint was cancer and cancer mortality, infection and infection mortality.
Main results
- Over 48 months, treatment with canakinumab (all doses) did not affect levels of LDL-c, HDL- and triglycerides.
- Up to 48 months, canakinumab lowered hsCRP levels: up to about 45% with 50mg SC q3mo, and up to about 60% with 150mg q3mo and 300mg q3mo and about 20% with placebo. The reduction of hsCRP was seen from the first measurement at 3 months and stable throughout the study period.
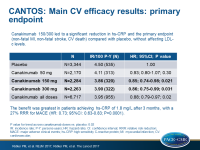
- The primary MACE endpoint was reduced statistically significantly by 15% in those treated with canakinumab 150/300 SC q3mo as compared with placebo (HR: 0.85, 95%CI: 0.76-0.96, P=0.007). This group showed 39% reduction in hsCRP. MACE+ was reduced by 17% (P=0.0006) and 30% reduction in need for revascularization procedures were seen as compared with placebo (P<0.0001).
- In those with reductions in hsCRP ≥median at 3 months (1.8 mg/L), a reduction of 27% in MACE+ was seen (HR: 0.73, 95%CI: 0.63-0.83, P=0.0001), while in those with hsCRP < median no significant reduction was seen (HR: 0.95, 95%CI: 0.84-1.08, P=0.47).
- Concerning safety results, more leukopenia was seen (0.30, 0.37 and 0.52 per 100 person years (PY) of exposure for 50, 150 and 300 mg respectively) with canakinumab as compared with placebo (0.24 per 100 PY, P for trend=0.002).
- The rate of any infection did not differ significantly between canakinumab (3.03, 3.13 and 3.25 per 100 PY) and placebo (2.86 per 100 PY). Fatal infections were seen more often with canakinumab (0.27, 0.28 and 0.30 vs. 0.18 per 100 PY, P for trend=0.09, P=0.02 for combined doses vs. placebo).
- In exploratory analyses, fewer fatal malignancies were seen with canakinumab (0.55, 0.50 and 0.31 vs. 0.64, P=0.0007).
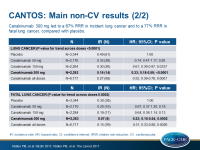
- Significantly fewer arthritis (2.15, 2.17 and 2.47 vs. 3.32, P=0.002), osteoarthritis (1.21, 1.12, 1.30 vs. 1.67, P=0.04) and gout (0.43, 0.35 and 0.37 vs 0.80, P=0.0001) were seen with canakinumab vs. placebo.
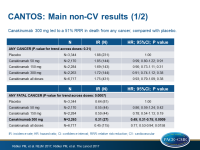
- With canakinumab 300 mg, 51% reduction in death from any cancer was seen as compared with placebo (HR: 0.49, 95%CI: 0.31-0.75, P=0.0009). With 150 mg, HR was 0.78 (95%CI: 0.54-1.13, P=0.19) and with 50 mg HR was 0.86 (95%CI: 0.59-1.24, P=0.24). P for trend across groups = 0.0007.
- The reduction in cancer mortality could mostly be ascribed to reduction in incident lung cancer: 300 mg: HR: 0.33, 95%CI: 0.18-0.59, P=0.00008, 150 mg: HR: 0.61, 95%CI: 0.39-0.97, P=0.34, 50 mg: HR: 0.77, 95%CI: 0.49-1.20, P=0.25. P for trend across groups = 0.0003.
- A reduction of 77% in fatal lung cancer was seen in the 300 mg group (HR: 0.23, 95%CI: 0.10-0.54, P=0.0002). With 150 mg, HR was 0.64 (95%CI: 0.36-1.14, P=0.13) and with 50 mg, HR was 0.71 (95%CI: 0.40-1.26, P=0.24). P for trend across groups was 0.0002.
Conclusion
The CANTOS trial data demonstrate that targeting the IL-1β to IL-6 pathway of innate immunity with canakinumab reduces CV event rates and potentially reduces rates of incident lung cancer and lung cancer mortality. These data provide proof that inflammation inhibition, in the absence of lipid lowering, can improve atherothrombotic outcomes and potentially alter the progression of some fatal cancers.
Ridker presented a concept of personalized predictive medicine, in which patients with known CV disease with elevated LDL and elevated hsCRP receive a high-intensity statin and are subsequently evaluated for having residual cholesterol risk, when LDL remains high but hsCRP is acceptable, or for having residual inflammatory risk, when LDL is acceptable but hsCRP is still high. These patients can receive additional LDL-c reduction or additional inflammation reduction, respectively.
In the discussion during the press conference, it was asked whether there is a clear-cut division between these groups. Ridker noted that naturally, there will be overlap between the phenotypes, but he wanted to show that the biology driving the outcomes is not the same in the groups. Also, he noted that the patients enrolled in this study are different from those enrolled in lipid-lowering trials like FOURIER and SPIRE.
Ridker was also asked whether he could envisage canakinumab as an add-on to statins, or could canakinumab be used as a stand-alone therapy. Ridker called statins the ‘miracle drug of the century’, so he could not imagine it likely that canakinumab would be used as monotherapy. These patients are very high risk patients, so they generally are on statins and other drugs.
The results show that not everybody shows benefit of treatment with canakinumab, so Ridker suggested that patients are tested after 3 months, to see if they show a reduction in hsCRP. Those who respond to treatment, can continue using canakinumab, and those who do not show lower hsCRP can stop, as they did not show clinical benefit in the trial. Efforts have been made to identify responders earlier, but this has proven difficult, so it might be better to start treatment and look at the biology.
Professor Peter Libby (Boston, MA, USA) was also present at the press conference and he added that inflammation is not necessarily in the plaque itself; CRP is an overall measure that drives atherogenic processes and complications. Traditional risk factors predominantly impact the biology of the arterial wall.
Regarding the cancer data, Ridker noted that cancer at baseline was an exclusion criterium. He thinks that there may have been small undiagnosed tumors, but equally distributed between groups. Canakinumab may slow progression of these small tumors.
It is anticipated that this trial will mean the start of a series of studies that explore inflammatory targets in the reduction of CV risk. In parallel, the NIH-funded CIRT trial is evaluating the effect of low-dose methotrexate on CV events.
- Our reporting is based on the information provided at the ESC congress -