Low dose NOAC plus aspirin improves outcomes in stable CV disease compared to aspirin alone
ESC 2017 Positive results for COMPASS trial, in which CV death, stroke or myocardial infarction risk are improved with NOAC rivaroxaban plus aspirin, as well as for COMPASS-PAD trial.
ESC 2017 - BarcelonaNews - Aug. 27, 2017
Rivaroxaban plus aspirin improves outcomes in stable cardiovascular disease (COMPASS)
Presented at the ESC congress 2017 by: John William Eikelboom (Hamilton, ONT, Canada)
Background
Aspirin is widely used for secondary prevention of cardiovascular (CV) diseases. A reduction of mortality in patients with a recent acute coronary syndrome (ACS) was also shown when using the non-vitamin K anttagonist (NOAC) rivaroxaban compared to placebo on a background of single or dual antiplatelet therapy (ATLAS ACS-2 TIMI 51 trial). Although rivaroxaban was accompanied with major bleedings, fewer were seen as compared with the anticoagulant warfarin.
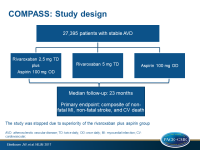
The COMPASS trial (Cardiovascular Outcomes for People Using Anti-coagulation Strategies) evaluated the effect on CV death, stroke or myocardial infarction (MI) (primary composite endpoint) in patients with stable coronary or peripheral artery disease (PAD), when using this combination of rivaroxaban and aspirin compared to rivaroxaban or aspirin alone. In this trial, 9 152 patients received 2.5 mg rivaroxaban twice a day plus 100 mg aspirin once a day, 9 177 patients received 5 mg rivaroxaban twice a day, and 9 126 patients received aspirin 100 mg once a day.
Main results
Because of the clear superiority of the combination of rivaroxaban and aspirin, both monotherapy arms with rivaroxaban or aspirin alone were stopped early. Therefore, median follow up was 23 months (anticipated follow-up duration 3-4 years).
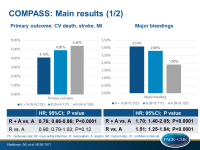
- The primary composite endpoint was associated with a 24% risk reduction when using the combination versus aspirin (HR 0.76, 95%CI 0.66-0.86, P<0.0001). Risks with rivaroxaban or aspirin alone were comparable (HR 0.90, 95%CI 0.79-1.03, P=0.12).
- Risk of major bleeding was enhanced with the combination compared to aspirin alone (HR 1.70, 95%CI 1.40-2.05, P<0.0001), however most were in the stomach or lower bowel and not serious: there was no significant increase in fatal or brain bleeding.
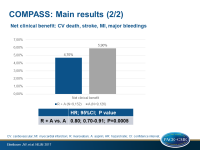
- The net clinical benefit (primary + severe bleeding events) of the combination was associated with an HR of 0.80, (95%CI 0.70-0.91, P=0.0005).
- The data indicate that for every 1000 patients treated for an average of 23 months, rivaroxaban plus aspirin prevents 13 heart attacks, strokes or CV deaths, and seven deaths from any cause, at a cost of 12 major bleeds, most of which were readily treatable.
Conclusion
The combination rivaroxaban plus aspirin improves survival and reduces CV death, stroke or MI in patients with stable coronary disease. Combination therapy gave benefit for the key composite outcome, meaning all clinically relevant outcomes including major complications. Rivaroxaban monotherapy did not reduce CV events as compared with aspirin.
John Eikelboom noted that this regimen should not be compared with dual antiplatelet therapy, which is given on the shorter term post-ACS. This study focused on patients receiving aspirin for long-term secondary prevention.
Eikelboom said in a press release “the substantial benefits seen with rivaroxaban and aspirin support the approach of using low doses of the two treatments in combination. Recent trials in other disease areas have demonstrated substantial benefits from using low doses of a combination of drugs and this concept is now further supported by the results of COMPASS”.
In addition to these results of patients with coronary stable disease, this combination was tested in 7 470 PAD patients (COMPASS-PAD) for whom no pharmacological therapy has been shown to clearly reduce risk for major adverse CV events (MACE) or limb events (MALE) yet. These data were presented by Sonia Anand (Hamilton, ONT, Canada).
Also in these patients, the combination significantly reduced risk for MACE and MALE, including major amputation. Additionally, regarding MALE, event rate was highest for patients treated with aspirin alone, intermediate for rivaroxaban alone and lowest with the combination, compared to aspirin. Major bleedings were non-significantly increased for fatal or critical organ bleeding.
During the discussion in the press conference, Anand noted that in PAD, guidelines recommend single therapy as first line option, since dual therapy has not proven efficacy.
Results of the COMPASS-PAD trial will be published in the Lancet.
- Our reporting is based on the information provided at the ESC congress -