PCSK9 inhibition reduces atherogenic lipid levels in T2DM patients with mixed dyslipidemia
In type 2 diabetic patients with mixed dyslipidemia on maximal statin therapy, the addition of the PCSK9 inhibitor alirocumab is more effective than usual care.
Alirocumab versus usual lipid-lowering care as add-on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: The ODYSSEY DM-DYSLIPIDEMIA randomized trialLiterature - Ray KK, Leiter LA, Müller-Wieland D, et al. - Diab Obes Metabol 2018; published online ahead of print
Background
Mixed dyslipidemia increases the CV risk of patients with type 2 diabetes (T2DM) and is characterized by elevated triglycerides (TGs), low levels of high-density lipoprotein cholesterol (HDL-c), while low-density lipoprotein cholesterol (LDL-c) may be normal [2,3]. It is not known whether proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibition is beneficial in this context.
In the ODYSSEY DM-DYSLIPIDEMIA trial, the efficacy and safety of alirocumab versus usual lipid-lowering care (UC) was assessed in high-risk T2DM patients with mixed dyslipidemia.
ODYSSEY DM-DYSLIPIDEMIA was a randomized, open-label, parallel group, multi-center trial, which included individuals, aged ≥18 years, with T2DM and mixed dyslipidemia, whose non-HDL-c was not adequately controlled despite maximally tolerated statin therapy for ≥4 weeks, without other lipid-lowering therapy, or who were statin-intolerant, and who had either a documented history of CVD or at least 1 additional CV risk factor, and a glycated hemoglobin (A1C) level <9% [13].
413 patients were included in this analysis. Mixed dyslipidemia was defined as non-HDL-c ≥2.59 mmol/L (≥100 mg/dL) and TGs ≥1.70 mmol/L (150 mg/dL) but <5.65 mmol/L (500 mg/dL). CVD was defined as coronary heart disease, peripheral arterial disease or ischemic stroke. Participants were stratified based on the need not to add lipid-lowering therapy or add lipid-lowering therapy that included fenofibrate, omega-3-fatty acids, ezetimibe, nicotinic acid, and were randomized in a 2:1 ratio to receive additionally either open-label alirocumab 75/150 mg every 2 weeks (Q2W), or UC for 24 weeks. The primary efficacy endpoint was the percentage change in non-HDL-c from baseline to week 24.
Main results
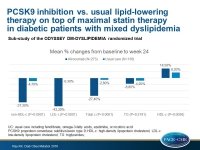
The mean percentage changes from baseline to week 24 with alirocumab vs. UC were:
- non-HDL-c: -37.3 (SE: 3.0)% vs. –4.7 (SE: 3.3)%; –32.5% difference; P<0.0001
- LDL-c: -43.3% vs. -0.3%; -43% difference; P<0.0001
- Total cholesterol: -27.4% vs. -2.8%; -24.6% difference; P<0.0001
- TGs: -13.0% vs. -8.8%; -4.2% difference; P=0.2191
- HDL-c: +14.5% vs. +8.2%; 6.2% difference; P=0.0026
- ApoB: -33.8% vs. -1.6%; -32.3% difference; P<0.0001
- Lp(a): -23.7% vs. +3.7%; -27.4% difference; P<0.0001
- LDL-p number: -41.6% vs. -3.9%; -37.8% difference; P<0.0001
- LDL-p size: -1.5% vs. +0.3%; -1.8% difference; P<0.0001
Results for the UC strata were similar to the overall analysis.
At week 24, more than two-thirds of alirocumab-treated individuals achieved levels of non-HDL-c <2.59 mmol/L (<100 mg/dL), LDL-c <1.81 mmol/L (<70 mg/dL) and ApoB <80 mg/dL. In addition, a greater proportion of individuals in the alirocumab group versus UC achieved a reduction in LDL-c from baseline of ≥50% (55.2% vs 3.8%).
Conclusion
In individuals with T2DM and mixed dyslipidemia who are not adequately controlled despite maximally tolerated statin therapy, the addition of the PCSK9 inhibitor alirocumab reduced more effectively their atherogenic lipid levels compared with the usual lipid-lowering therapeutic approaches.
References
1. Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia 2015;58:886-899
2. Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, prediabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34:3035-3087
3. Müller-Wieland D, Leiter LA, Cariou B, et al. Design and rationale of the ODYSSEY DM-DYSLIPIDEMIA trial: lipid-lowering efficacy and safety of alirocumab in individuals with type 2 diabetes and mixed dyslipidaemia at high cardiovascular risk. Cardiovasc Diabetol 2017;16:70