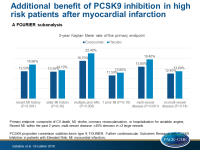
Greater risk reduction with PCSK9 inhibition in readily ascertainable high risk patient subgroups
Patients with a history of MI who are closer to their most recent event, have had multiple prior MIs or have residual multi-vessel CAD, had higher CV risk reductions with evolocumab.
Clinical Benefit of Evolocumab by Severity and Extent of Coronary Artery Disease: An Analysis from FOURIERLiterature - Sabatine MS, De Ferrari GM, Giugliano RP, et al. - Circulation. 2018; published online ahead of print
Introduction and methods
The Further cardiovascular Outcomes Research with PCSK9 Inhibition in patients with Elevated Risk (FOURIER) trial showed that the PCSK9 antibody evolocumab, when added to statin therapy, lowered LDL-c by 59%, and significantly reduced the risk of cardiovascular (CV) events in patients with stable atherosclerotic CV disease, the majority of whom (N= 22,351) had a history of myocardial infarction (MI) [1-4].
This analysis of the FOURIER trial aimed to assess whether common and readily ascertainable features would identify subsets of patients that derive greater clinical risk reduction with evolocumab, for instance based on the timing from the most recent MI, the number of prior MIs, and the presence of residual multi-vessel coronary artery disease (CAD), defined as ≥40% stenosis in ≥2 large vessels.
FOURIER enrolled 27,564 patients aged 40-85 years with clinically evident atherosclerotic CV disease, and additional CV risk factors, as well as LDL-c ≥70 mg/dL or non-HDL-c ≥100 mg/dL while taking an optimized lipid-lowering. Patients received subcutaneous evolocumab (either 140 mg Q2W or 420 mg Q4W) or matching placebo, and were followed for a median of 2.2 years (IQR: 1.8-2.5 years). The primary endpoint of FOURIER was the composite of CV death, MI, stroke, coronary revascularization, or hospitalization for unstable angina. The key secondary endpoint was the composite of CV death, MI, or stroke.
Main results
- More recent MI (within the past 2 years), multiple prior MIs, and residual multi-vessel CAD were independent predictors of the primary endpoint, with adjusted HRs of 1.37 (95%CI: 1.22-1.53), 1.78 (95%CI: 1.59-1.99) and 1.39 (95%CI: 1.24-1.56), respectively (all P<0.001).
- Evolocumab consistently lowered LDL-c by 59-61% regardless of time from most recent MI, number of prior MIs or presence of residual multi-vessel CAD, with median achieved LDL-c in the evolocumab arm of 29-30 mg/dL.
- The 3-year Kaplan Meier rate of the primary endpoint in patients with a more recent MI was 13.5% in the evolocumab group and 16.9% in the placebo group (HR: 0.80; 95%CI: 0.71-0.91; P<0.001). In patients for whom MI was ≥2 years ago, the respective rates were 13.3% and 14.1% (HR: 0.95; 95%CI: 0.85-1.05; P=0.30).
- The 3-year Kaplan Meier rate of the primary endpoint in patients with multiple prior MIs was 18.7% in the evolocumab group vs. 22.4% in the placebo group (HR: 0.82; 95%CI: 0.72-0.93; P=0.003), and in those with 1 previous MI it was 11.5% vs. 12.8% (HR: 0.92; 95%CI: 0.84-1.02; P=0.10).
- The 3-year Kaplan Meier rate of the primary endpoint in patients with multi-vessel disease was 15.8% in the evolocumab group vs. 19.4% in the placebo group (HR: 0.79; 95%CI: 0.69-0.91; P=0.001), and in patients without multi-vessel disease it was 12.4% vs. 13.6% (HR: 0.93; 95%CI: 0.85-1.02; P=0.14).
- The absolute risk reductions with evolocumab for the primary and key secondary endpoints in patients with any high-risk feature were 3.0% and 2.5%, respectively, while the corresponding values were -0.6% and 0.5% in patients without high-risk features.
Conclusion
Patients with a history of MI who are closer to their most recent event, have had multiple prior MIs or have residual multi-vessel CAD are at higher risk for major vascular events. The relative and absolute risk reductions in CV outcomes with evolocumab were greater in these high-risk subgroups. These data support the targeting of such high risk patients with PCSK9 inhibition.
References
1. Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J. 2016;173:94-101.
2. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376:1713-1722.
3. Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70:1785-1822.
4. Landmesser U, Chapman MJ, Stock JK, et al. 2017 Update of ESC/EAS Task Force on practical clinical guidance for proproteinconvertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur Heart J. 2017.