DPP-4 inhibitor is safe in post-ACS patients with T2DM, even in the high-risk period
Compared with placebo, alogliptin did not increase CV risk in type 2 diabetic patients up to 6 months after an ACS, or later.
Early and Chronic Dipeptidyl-Peptidase-IV Inhibition and Cardiovascular Events in Patients With Type 2 Diabetes Mellitus After an Acute Coronary Syndrome: A Landmark Analysis of the EXAMINE TrialLiterature - Sharma A, Cannon CP, White WB, et al. - J Am Heart Assoc.2018;7:e007649
Introduction and methods
Patients with type 2 diabetes mellitus (T2DM) are at particularly high risk of recurrent events and heart failure hospitalization (HHF) within the first 6 months after an acute coronary syndrome (ACS) [1-3]. CV safety of dipeptidyl dipeptidase-4 (DPP-4) inhibitors in T2DM patients from randomization to 6 months post-ACS or 6 months to end of follow-up, has not been adequately evaluated.
In this landmark analysis of the EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin Versus Standard of Care) trial, the burden of vascular and HF events post-ACS was assessed in the early (within 6 months) and late periods (after 6 months). Moreover, it was evaluated whether the risk of CV events, including HF, is associated with early (within 6 months) and late (after 6 months) DPP-4 inhibition with alogliptin.
EXAMINE [4,5], a double-blind, placebo-controlled, non-inferiority trial, enrolled 5380 stable T2DM patients 15-90 days post-ACS, who were randomly assigned to receive alogliptin or placebo. Patients with end-stage renal disease on dialysis, NYHA class IV HF, refractory angina, uncontrolled arrhythmias, significant valvular heart disease, or severe uncontrolled hypertension were excluded. The median follow-up time was 18 months. The combined primary endpoint was the time to CV death or myocardial infarction (MI) or stroke. Additional endpoints included revascularization for unstable angina (RUA) and HHF.
Main results
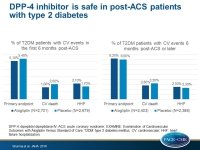
Within the first 6 months post-ACS, 5.3% of patients experienced the primary endpoint compared with 8.1% in the late phase, whereas 7.8% experienced the composite endpoint of all-cause mortality/non-fatal MI/non-fatal stroke/RUA/HHF within the first 6 months post-ACS compared with 11.6% in the late period.
Compared with placebo:
- alogliptin did not increase the risk of the primary endpoint neither in the early (5.1% vs. 5.4%; HR for first 6 months: 0.96, 95%CI: 0.76–1.21), nor in the late phase of the analysis (8.2% vs. 8.0%; HR after first 6 months: 1.03; 95%CI: 0.84–1.26)
- alogliptin decreased numerically, but not significantly, the risk of CV death both in the early (1.6% vs. 2.0%; HR: 0.82; 95%CI: 0.55–1.23), as well as in the late phase of the study (2.8% vs. 3.2%; HR: 0.88; 95%CI: 0.63–1.21)
- the risk of HHF was numerically but not significantly increased with alogliptin in the early (2.1% vs. 1.7%; HR: 1.23; 95%CI: 0.84–1.82) and late study period (2.5% vs. 2.3%; HR: 1.1; 95%CI: 0.76–1.59)
- the risk of death/MI/stroke/RUA/HHF was not increased in the early (HR: 0.96; 95%CI: 0.79–1.16), nor in the late phase of the study (HR: 1.64; 95%CI: 0.88–1.23) with alogliptin
- the risks of non-fatal MI, non-fatal stroke, and all-cause mortality were not increased by alogliptin in the early or late study period
Conclusion
Compared with placebo, alogliptin did not increase the CV risk in T2DM patients from randomization to 6 months post-ACS, or later. Because T2DM patients experience a large burden of clinical events in the early period following ACS, further studies are needed to ensure the safety of antihyperglycemic therapies initiated during this time period.
References
1. Jernberg T, Hasvold P, Henriksson M, et al. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J. 2015;36:1163–1170.
2. Pilgrim T, Vranckx P, Valgimigli M, et al. Risk and timing of recurrent ischemic events among patients with stable ischemic heart disease, non-ST-segment elevation acute coronary syndrome, and ST-segment elevation myocardial infarction. Am Heart J.2016;175:56–65.
3. Roffi M, Patrono C, Collet J-P, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J.2016;37:267–315.
4. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med.2013;369:1327–1335.
5. White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J.2011;162:620–626.