Direct thrombin inhibitor reduces vascular complications in patients with myocardial injury after non-cardiac surgery
Dabigatran therapy was associated with a lower risk of major vascular complications without an increase in major bleeding in patients with myocardial injury after non-cardiac surgery.
Dabigatran in patients with myocardial injury after non-cardiac surgery (MANAGE): an international, randomised, placebo-controlled trialLiterature - Devereaux PJ, Duceppe E, Guyatt G, et al. - The Lancet 2018; 391: 2325–34
Introduction and methods
Myocardial injury after non-cardiac surgery (MINS), defined as myocardial infarction (MI) or troponin elevation within 30 days after surgery, is independently associated with an increased risk of CV complications and death in the first two years after surgery [1,2]. Dabigatran, an oral direct thrombin inhibitor, reduces the risk of thrombotic events in patients with MI and vascular disease [3,4], but it is not known whether it prevents vascular complications in patients with MINS.
In the investigator-initiated, international, randomized, placebo-controlled MANAGE trial [5], the potential of dabigatran to prevent major vascular complications in patients with MINS was evaluated. In the MANAGE trial, patients, aged ≥45 years, who had elevated troponin with ischemic signs or symptoms, ischemic electrocardiographic changes, new or presumed new ischemic abnormality on cardiac imaging, or isolated elevated troponin without an alternative explanation to ischemic myocardial injury after non-cardiac surgery were included. Patients with a hemorrhagic disorder or those who required anticoagulation due to prosthetic heart valve, venous thromboembolism, or atrial fibrillation, as well as patients with a contraindication to anticoagulation therapy and those with an estimated glomerular filtration rate <35 mL/min were excluded from the study.
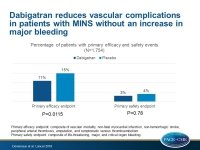
Patients were randomized in a 1:1 fashion to receive dabigatran 110 mg orally twice daily or placebo, and patients not taking a proton-pump inhibitor were also randomly assigned (1:1) to omeprazole 20 mg orally once daily or placebo. Patients were followed up for a maximum of two years. The primary efficacy outcome was a composite of vascular mortality, non-fatal MI, non-hemorrhagic stroke, peripheral arterial thrombosis, amputation, and symptomatic venous thromboembolism. The primary safety outcome was a composite of life-threatening, major, and critical organ bleeding.
Main results
- Out of a total of 1,754 patients, 91% had a MINS event, 46% discontinued the study drug permanently in the dabigatran group, and 43% in the placebo group.
- The primary efficacy outcome occurred in 11% of patients in the dabigatran group and in 15% of patients in the placebo group (HR: 0.72; 95%CI: 0.55–0.93; P=0.0115).
- Dabigatran did not increase the primary safety outcome compared with placebo (3% vs. 4%; HR: 0.92; 95%CI: 0.55–1.53; P=0.78).
- Omeprazole had no significant effect on the results of the dabigatran primary safety analysis (Pinteraction=0.37).
Conclusion
Myocardial injury was identified by troponin screening in the majority of patients who had undergone non-cardiac surgery. These results suggest that routine troponin measurement is justified in patients undergoing non-cardiac surgery. Anticoagulation therapy with dabigatran was associated with a lower risk of major vascular complications in these patients, without an increased risk of major bleeding.
References
1. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120: 564–78.
2. Devereaux PJ, Biccard BM, Sigamani A, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017; 317: 1642–51.
3. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370: 949–56.
4. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost 2011; 105: 721–29
5. Duceppe E, Yusuf S, Tandon V, et al. Design of a randomized placebo-controlled trial to assess dabigatran and omeprazole in patients with myocardial injury after noncardiac surgery (MANAGE). Can J Cardiol 2018; 34: 295–302.