LDL-c reductions below 1.6 mmol/L provide additional vascular risk benefit
In a meta-analysis of statins and non-statins, LDL-c reductions reaching to a median of 0.5 mmol/L (21 mg/dL) in patients starting with median LDL-c levels of 1.6-1.8 mmol/L (63 mg/dL) are associated with lower risk of major vascular events.
Efficacy and Safety of Further Lowering of Low-Density Lipoprotein Cholesterol in Patients Starting With Very Low Levels: A Meta-analysisLiterature - Sabatine MS, Wiviott SD, Im KA et al. - JAMA Cardiol 2018; published online ahead of print
Introduction and methods
The Cholesterol Treatment Trialists Collaboration (CTTC), a meta-analysis of 26 statin trials, demonstrated a 22% relative risk reduction in major vascular events (MVE) per 1 mmol/L (38.7 mg/dL) reduction in LDL-c, including patients with baseline LDL-c levels as low as ≤2 mmol/L (77.3 mg/dL) [1]. However, it is not clear whether this clinical benefit applies also to patients with baseline LDL-c levels below that.
This meta-analysis evaluated the efficacy and safety of lowering LDL-c levels in patients with median LDL-c levels ≤1.8 mmol/L (70 mg/dL). The CTTC was used to evaluate this objective in patients receiving statins. Trials with other lipid-lowering medications (OLLM), given on top of statins, were assessed through a literature search and meta-analysis, which was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [2]. Eligible for the OLLM meta-analysis were randomized, double-blind, controlled outcome studies including patients with mean or median LDL-c levels ≤1.8 mmol/L.
For each trial, the risk ratio (RR) per 1 mmol/L difference in LDL-c between treatment arms was calculated. A fixed-effects inverse-weighting model was used to assess the results. The association between achieved LDL-c and estimated 5-year rate of MVE (including coronary heart death, myocardial infarction, ischemic stroke, or coronary revascularization) was evaluated.
Main results
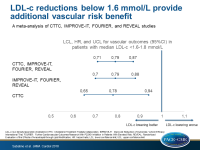
The literature search identified three eligible OLLM secondary prevention trials: IMPROVE-IT [3], FOURIER [4] and REVEAL [5].
In a subgroup of patients with a mean LDL-c level of 1.7 mmol/L (median: 1.6-1.8 mmol/L) in the control arm (baseline LDL-c for the experimental group was not reported), the RR for MVE per 1 mmol/L LDL-c reduction was:
- in the three OLLM studies: 0.79 (95%CI: 0.70-0.88; P <0.001)
- in the CTTC: 0.78 (95%CI: 0.65-0.94)
- in all studies combined: 0.79 (95%CI: 0.71-0.87; P <0.001)
In the OLLM studies, the significant association between LDL-c levels and MVE persisted until LDL-c levels reached 0.5 mmol/L (21 mg/dL) (β: 0.35; 95%CI: 0.25-0.45; P <0.001).
There was no increased risk of serious adverse events, myalgias and/or myositis, elevation in the level of aminotransferases, new onset diabetes, hemorrhagic stroke, or cancer in any of the trials individually or when meta-analyzed.
Conclusion
LDL-c reductions reaching a median of 0.5 mmol/L (21 mg/dL) in patients with median LDL-c levels of 1.6-1.8 mmol/L (63 mg/dL) in the control arm are associated with a risk reduction in MVE, without an accompanying increased risk of adverse events. The risk of MVE was reduced by 21% per 1 mmol/L LDL-c reduction, which was of the same magnitude as that observed in the CTCC with starting LDL-c levels almost twice as high.
Editorial comment
In his editorial article, Gotto [6] discusses the study published by Sabatine et al., which he characterizes as being ‘extremely well done’. He notes that these data provide a solid reason to update the current American Heart Association/American College of Cardiology 2013 guidelines, which may not recommend the optimal risk reduction achievable, and he concludes: ‘Whether one calls it a target or a threshold, practicing physicians need some guidance as they venture into achieved levels of LDL-C levels that are as foreign as travel to outer space. I have confidence that the new guidelines will be closer to a global positioning system map rather than just a compass and the stars. Treating physicians should apply informed clinical judgment to each individual patient.’
References
References
1. Baigent C, Blackwell L, Emberson J, et al; Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: ameta-analysis of data from 170,000 participants in 26 randomised trials. Lancet.2010;376(9753):1670-1681.
2. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Ann Intern Med.2009;151(4):264-269, W64.
3. Cannon CP, BlazingMA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397.
4. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722.
5. Bowman L, Hopewell JC, Chen F, et al; HPS3/TIMI55–REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217-1227. 6. Gotto AM. Low-Density Lipoprotein Cholesterol and Cardiovascular Risk Reduction. How Low Is Low Enough Without Causing Harm? JAMA Cardiol 2018; published online ahead of print.