GLP-1 receptor agonist in combination with SGLT2 inhibitor better than monotherapy in diabetic patients
In adult T2DM patients, the combination of exenatide and dapagliflozin led to significantly better glycemic control and reductions in weight and SBP compared with monotherapy after 52 weeks.
Safety and Efficacy of Exenatide Once Weekly Plus Dapagliflozin Once Daily Versus Exenatide or Dapagliflozin Alone in Patients With Type 2 Diabetes Inadequately Controlled With Metformin Monotherapy: 52-Week Results of the DURATION-8 Randomized Controlled TrialLiterature - Jabbour SA, Frias JP, Hardy E et al. - Diabetes Care 2018; published online ahead of print
Introduction and methods
Medical guidelines recommend glucose-lowering drug combinations with different modes of action for the treatment of type 2 diabetes (T2DM) [1-3]. DURATION-8, a multicenter, double-blind, randomized, phase 3 trial assessed the effects of the combination therapy of exenatide and dapagliflozin in 695 adult T2DM patients on metformin therapy (≥1,500 mg/day) with poor glycemic control, defined as glycated hemoglobin (HbA1c) between 8.0–12.0% [4]. Exenatide is a glucagon-like peptide 1 receptor agonist (GLP-1RA) administered subcutaneously once weekly (2 mg), and dapagliflozin is a sodium–glucose cotransporter 2 (SGLT2) inhibitor, administered in 10 mg tablets once daily. After 28 weeks of treatment, the combination therapy led to significantly greater improvements in glycemic control, weight, and systolic blood pressure (SBP) compared with exenatide or dapagliflozin alone.
In this analysis of the first 24-week extension of DURATION-8, during which patients continued to receive their randomized treatment, the durability of the efficacy and safety of exenatide plus dapagliflozin was evaluated at a total of 52 weeks of therapy.
The primary end point of the DURATION-8 study was the change in HbA1c from baseline to week 28. At week 52 all end points were exploratory, including change from baseline of HbA1c, of fasting plasma glucose (FPG), of 2-h postprandial glucose (PPG) during a standardized liquid meal test, change in six-point self-monitored blood glucose, the proportion of patients achieving glycemic targets (HbA1c <7.0% or ≤6.5%), change in from baseline for several CV risk factors including SBP and the proportion of patients with weight loss of ≥5%.
Main results
- At week 52, the mean HbA1c reductions from baseline were 1.75% (SD:0.10) in the combination therapy group, 1.38% (SD:0.10) in the exenatide group, and 1.23% (SD:0.10) in the dapagliflozin group (between-group difference with 95%CI: vs exenatide -0.37 (-0.64 to -0.11); P = 0.006 and vs dapagliflozin -0.52 (-0.79 to -0.26); P<0.001).
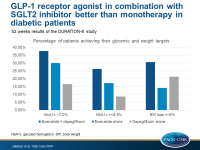
- At week 52, mean HbA1c was 6.87% in the combination therapy group, 7.21% in the exenatide group, and 7.36% in the dapagliflozin group.
- At week 52, an HbA1c target <7.0% was achieved by 37.7% of patients in the combination therapy group, by 30.0% of patients in the exenatide group, and in 16.5% of patients in the dapagliflozin group, whereas the HbA1c target of ≤6.5% was achieved by 26.3% of patients in the combination therapy group, by 17.2% of patients in the exenatide group and by 8.7% of patients in the dapagliflozin group.
- At week 52, significantly greater mean reductions in FPG, in 2-h PPG, and in mean six-point self-monitored blood glucose concentrations were observed in the combination therapy group compared with the exenatide group or the dapagliflozin group.
- At 52 weeks, 30.7% (24.7–36.7%) of patients in the combination therapy group, 14.1% (9.6–18.6%) of patients in the exenatide group, and 21.3% (16.0–26.6%) in the dapagliflozin group achieved a weight loss of ≥5% (between-group difference: vs exenatide 16.6; P<0.001, vs dapagliflozin 9.4; P=0.022).
- At week 52, significantly greater mean reductions in SBP were observed in the combination therapy group (-4.5 [SD: 0.8]) compared with the exenatide group (-0.7 [SD: 0.9]) or the dapagliflozin group (-2.7 [SD: 0.8]) (between-group difference: vs exenatide -3.9 (-6.1 to -1.7); P<0.001), vs dapagliflozin -1.8 (-3.9 to 0.3); P=0.100), whereas there were no significant differences between groups in regard to diastolic blood pressure, waist circumference, and fasting cholesterol levels.
- Combination therapy was well tolerated and across all treatment groups comparable proportions of patients experienced mild or moderate adverse effects over 52 weeks.
Conclusion
In adult T2DM patients on metformin therapy with poor glycemic control, the combination of exenatide and dapagliflozin led to significantly greater reductions in parameters of glycemic control, body weight, and SBP over 52 weeks, compared with each drug alone and this combination therapy was well tolerated.
References
1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149
2. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm –2017 executive summary. Endocr Pract 2017;23:207–238
3. American Diabetes Association. Pharmacologic approaches to glycemic treatment. Sec. 8. In Standards of Medical Care in Diabetesd2018. Diabetes Care 2018;41(Suppl. 1):S73–S85
4. 10. Frıas JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:1004–1016