PCSK9 inhibition results in consistent LDL-c lowering benefit in various patient subgroups
In PROFICIO, a pooled analysis of four phase 3 trials showed significantly greater reductions in LDL-c with evolocumab vs placebo or ezetimibe for all demographic and disease status subgroups.
Consistent LDL-C response with evolocumab among patient subgroups in PROFICIO: a pooled analysis of 3146 patients from phase 3 studiesLiterature - Stroes E, Robinson JG, Raal FJ et al. - Clin Cardiol 2018; published online ahead of print
Introduction and methods
The Program to Reduce low density lipoprotein cholesterol (LDL-c) and Cardiovascular Outcomes Following Inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) In Different Populations (PROFICIO) trial tested efficacy and safety of evolocumab in different patient populations with hypercholesterolemia, including those with familial hypercholesterolemia or statin intolerance. Within each of the studies in the PROFICIO program, evolocumab therapy substantially and consistently decreased LDL-c [1-5].
This analysis pooling data from four randomized studies further investigated the LDL-c reduction associated with evolocumab therapy in different patient subsets based on demographic and disease characteristics.
This pooled analysis included four placebo- or ezetimibe-controlled, phase 3 trials and evaluated the difference in percent change from baseline in LDL-c between each evolocumab dosing regimen and control therapy, using the mean of week 10 and 12 LDL-c values. Background lipid therapies included statin monotherapy or statin combined with ezetimibe. The evolocumab dosing regimens were 140 mg subcutaneously every two weeks (Q2W) or 420 mg monthly (QM).
In total, 3,146 patients with primary hypercholesterolemia and cardiovascular (CV) risk of various levels, familial hypercholesterolemia, and prior intolerance to ≥2 statins were randomized and received at least one dose of evolocumab or control therapy.
Main results
In the pooled population, the mean age of participants was 57.8 years, 49.4% of patients were women, 91.5% were white, and 54.1% were receiving statins.
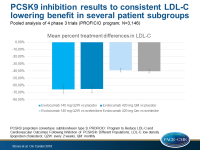
In the pooled analysis, the mean percent treatment differences from baseline in LDL-c were:
- for evolocumab 140 mg Q2W vs placebo: –65.7% (95%CI: –70.9 to –60.6)
- for evolocumab 420 mg QM vs placebo: –65.0% (95%CI: –69.5 to –60.4)
- for evolocumab 140 mg Q2W vs ezetimibe: –38.9% (95%CI: –41.3 to – 36.4)
- for evolocumab 420 mg QM vs ezetimibe: –40.3% (95%CI: –42.6 to –38.0)
LDL-c changes were similar in the following subgroups (consistent after adjusting for age, body mass index, baseline LDL-c, baseline PCSK9, and baseline statin treatment):
- males and females,
- individuals <65 and ≥65 years,
- individuals of different races and ethnicities
- individuals with different glucose tolerance status
- individuals in CV risk categories,
Overall, adverse events foreseen with the evolocumab 140 mg Q2W and 420 mg QM dosing regimens were similar to those seen in the control arms.
Conclusion
In a pooled analysis of four phase 3 trials, evolocumab 140 mg Q2W and 420 mg QM resulted in significantly greater reductions in LDL-c vs placebo or ezetimibe, on top of background statin therapy for all demographic and disease status subgroups analyzed, with similar adverse effects compared to controls.
References
1. Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809-19.
2. Giugliano RP, Desai NR, Kohli P, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007-17.
3. Hirayama A, Honarpour N, Yoshida M, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk--primary results from the phase 2 YUKAWA study. Circ J. 2014;78:1073-82.
4. Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531-40.
5. Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995-2006.