Glucagon-like peptide-1 analogue promotes weight loss in obese, non-diabetic individuals
In obese individuals without diabetes, semaglutide significantly and dose-dependently reduced body weight compared with placebo and liraglutide, on top of healthy diet and exercise.
Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trialLiterature - O’Neil PM, Birkenfeld AL, McGowan B, et al. - The Lancet 2018; published online ahead of print
Introduction and methods
The human glucagon-like peptide-1 (GLP-1) analogues liraglutide and semaglutide have been developed for the treatment of type 2 diabetes. Both drugs modulate appetite and energy intake, and have a positive effect on weight management on top of exercise and diet in diabetic patients [1,2].
This is a randomized, double-blind, controlled, dose-ranging phase 2 study to evaluate the weight-loss efficacy and safety of semaglutide given once daily for 52 weeks compared with an active-control (liraglutide) and placebo, in obese individuals.
Eligible participants were ≥18 years, without diabetes, and with a self-reported stable (maximum ±5kg within 90 days) body-mass index (BMI) of ≥30 kg/m². Furthermore, they had at least one previous unsuccessful non-surgical weight-loss attempt, and were free of major depressive symptoms (defined as a screening Patient Health Questionnaire-9 [PHQ-9] score <15). They were randomized 6:1 (active:placebo) to receive either semaglutide at one of five doses (0.05 mg, 0.1 mg, 0.2 mg, 0.3 mg, or 0.4 mg) or liraglutide (3.0 mg) as once-daily subcutaneous injections, or placebo. For each active treatment group there was a matching placebo group.
Semaglutide was initiated at 0.05 mg per day and escalated to the next dosing level every 4 weeks, whereas liraglutide was initiated at 0.6 mg per day and escalated by 0.6 mg per week. Nutritional and physical activity counselling was provided by qualified research staff every 4 weeks. The primary endpoint was the relative percentage change in bodyweight from baseline to week 52. Pre-specified secondary endpoints included categorical weight loss of >5% or >10% of baseline.
Main results
- Out of 957 patients who were randomly assigned to study medication, 81% completed 52 weeks of treatment, and 19% discontinued treatment early, out of which 18% were on active treatment and 24% on placebo.
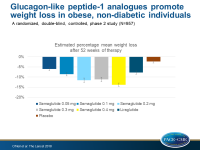
- The estimated mean weight reductions from baseline to week 52 for participants receiving semaglutide ranged from 6.0% (SE: 0.85) in the 0.05 mg group to 13.8% (SE: 0.83) in the 0.4 mg group, for participants receiving liraglutide they were 7.8% (SE: 0.85), and for those receiving placebo they were 2.3% (SE: 0.74).
- Patients on semaglutide (all doses) or liraglutide had significantly greater estimated body weight reductions compared with placebo at week 52, and these reductions remained significant after multiple adjustments for multiple testing in the groups escalated to final semaglutide dose.
- When comparing the effect of semaglutide with liraglutide, doses of semaglutide of 0.2 mg or more showed significantly greater weight loss at 52 weeks than liraglutide 3.0 mg.
- Estimated (logistic regression) and observed weight loss of at least 5%, 10%, 15% or 20% of baseline with semaglutide showed a dose-responsive pattern.
- In the post-hoc analyses, 9–50% of participants receiving semaglutide showed weight loss of 15% or more compared with 4% receiving placebo and 20% receiving liraglutide, and 5–35% lost 20% or more compared with 2% receiving placebo and 6% receiving liraglutide.
- Semaglutide was well tolerated, and the most common adverse events were dose-related gastrointestinal symptoms, primarily nausea.
Conclusion
In obese individuals without diabetes, semaglutide at doses of at least 0.2 mg per day, combined with diet and exercise modification, resulted in dose-dependent body weight loss compared with placebo and compared with liraglutide 3.0 mg.
References
1. Flint A, Raben A, Astrup A, et al. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101: 515–20.
2. Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 2007; 113: 546–93.