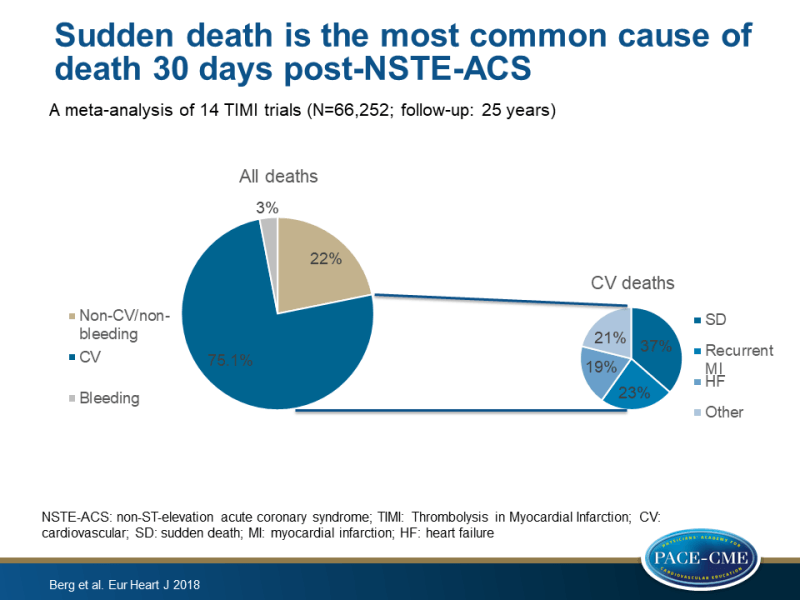
Sudden death is the most common cause of death after 30 days post-NSTE-ACS
In a population derived from 14 TIMI clinical trials, CV events caused the majority of deaths post-NSTE-ACS, with recurrent MI representing the most common cause of death within the first 30 days, and sudden death after the first 30 days.
Modes and timing of death in 66 252 patients with non-ST-segment elevation acute coronary syndromes enrolled in 14 TIMI trialsLiterature - Berg DD, Wiviott SD, Braunwald E et al. - Eur Heart J 2018; published online ahead of print
Introduction and methods
The early consequences of non-ST-elevation acute coronary syndrome (NSTE-ACS) are well known, but the long-term risks are less well described [1]. This analysis of 14 Thrombolysis in Myocardial Infarction (TIMI) Study Group clinical trials evaluated the modes and timing of death of NSTE-ACS patients.
NSTE-ACS patients were recruited between 1989 and 2014. All 14 trials contributed ≥10 deaths and had a median follow-up duration of ≥100 days. Death was classified as cardiovascular (CV), bleeding, or non-CV/non-bleeding. CV deaths were further subdivided into specific modes of death, including sudden death (SD), recurrent myocardial infarction (MI), heart failure (including cardiogenic shock), and other CV death (including ischemic stroke, pulmonary embolism, acute aortic syndromes, and cardiac rupture), according to the definitions of the American College of Cardiology/American Heart Association [2].
Main results
- A total of 66,252 patients were followed for a median time of 372 days (IQR: 218–521 days). The mode of death was classifiable in 82.8% of 3147 patients who died during follow-up.
- Kaplan–Meier estimates yielded death rates of 1.5% at 30 days (95%CI: 1.4–1.6%), 3.0% at 6 months (95%CI: 2.9–3.2%), 4.3% at 1 year (95%CI: 4.2–4.5%), and 6.5% at 2 years (95%CI: 6.3–6.8%).
- Out of the 2606 classifiable deaths, 75.1% were CV deaths, 3.0% were due to a fatal bleeding event, and 21.8% were non-CV deaths other than bleeding.
Modes of death at 1 year
- The crude cumulative incidences of CV death, fatal bleeding, and death from a non-CV/non-bleeding event were 2.67%, 0.11%, and 0.66%, respectively, at 1 year following trial enrolment.
- The most common modes of CV death were SD (36.4%) and recurrent MI (23.4%).
- The cumulative rates of SD, recurrent MI, heart failure, and other CV death were 0.90%, 0.67%, 0.54%, and 0.55%, respectively, at 1 year following trial enrolment.
Modes of death during and after the first 30 days
- The cumulative incidences of SD, recurrent MI, heart failure, and other CV death were 0.66%, 0.32%, 0.26%, and 0.28%, respectively, at 1 year minus the first 30 days following the qualifying NSTE-ACS event.
- CV deaths represented the majority of deaths during both the first 30 days following randomization and after 30 days, but were relatively more common during the 0–30-day period (84.5% vs. 70.0%; P<0.001).
- The proportions of CV deaths owing to recurrent MI and HF were greater in the first 30 days than later (30.6% vs. 18.7%; P<0.001 and 24.3% vs. 15.7%, P<0.001 respectively). The proportion of SD was lower in the first 30 days (21.6% vs. 46.2%; P< 0.001).
- The cumulative incidence of SD increased steadily and more rapidly than other modes of CV death after 30 days, and exceeded the rate of death due to recurrent MI by day 119.
Conclusion
In a population derived from 14 clinical trials, CV events caused the majority of deaths post-NSTE-ACS. Recurrent MI represented the largest proportion of CV deaths in the first 30 days following NSTE-ACS, whereas SD was the most frequent cause of death beyond 30 days. Further research should shed light on how CV risk can be modified following NSTE-ACS to improve survival in this population.
Editorial comment
In his editorial article, Lars Wallentin [3] contrasts the results of the meta-analysis published by Berg et al., with the recent results of the SWEDEHAERT (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry that reports a 3-4-fold higher total and CV mortality at 1 (5.4% and 4.4% vs. 1.4% and 1.2%, respectively) and 12 (14.3% and 9.9% vs. 4.3% and 2.9%, respectively) months compared to the TIMI results, probably due to differences in patient selection. SWEDEHEART included all-comers with suspected or definite NSTEMI immediately on arrival while the RCTs include selected survivors at a later stage of the disease. This emphasizes concerns for the relevance of the results when testing new therapies in very selected low-risk populations. The data obtained from a selected RCT might not be representative of the complex real-life population where effects might be both smaller and larger compared to the selected RCT populations. Further, there is the risk of losing information on both efficacy and safety with testing new therapies in lower risk populations, which might have important influences on the further development and eventual approval of new therapies.
The authors conclude: ‘In order to increase the chances of clear and relevant results, all trials should be encouraged as far as possible to include the whole target population. Such a strategy is both doable and very cost-effective by performing the RCTs embedded in continuous registries rather than using the conventional approach.’
References
1. Budaj A, Eikelboom JW, Mehta SR, et al; OASIS 5 Investigators. Improving clinical outcomes by reducing bleeding in patients with non-ST elevation acute coronary syndromes. Eur Heart J 2008;30:655–661.
2. Hicks KA, Tcheng JE, Bozkurt B, et al; American College of Cardiology, American Heart Association. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation 2015;132:302–361.
3. Wallentin L. Different perspectives on outcomes in patients with non-ST-elevation myocardial infarction when observed in clinical trials and in real life. Eur Heart J 2018; published online ahead of print.
