PCSK9-targeted siRNA therapy: effective, safe and durable lipid-lowering irrespective of diabetes status
A post-hoc analysis showed reduced LDL-c levels and improved lipid profiles up to 180 days with one or two doses of inclisiran on top of standard care in the presence and absence of diabetes, compared to placebo.
Inclisiran Lowers LDL-C and PCSK9 Irrespective of Diabetes Status: The ORION-1 Randomized Clinical TrialLiterature - Leiter LA, Teoh H, Kallend D et al. - Diabetes Care 2018: published online ahead of print
Introduction and methods
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition is associated with CV benefit in individuals with stable CVD [1,2] and recent acute coronary syndromes [3,4] independent of whether they have diabetes [2,3]. Inclisiran, a synthetic siRNA that drives PCSK9-specific mRNA degradation in the liver, has been reported to significantly lower LDL-c levels in individuals with ASCVD or ASCVD risk and high LDL-c levels in the ORION-1 trial [5,6].
The phase 2, multicenter, double-blind, placebo-controlled, multiple-ascending-dose ORION-1 trial randomized eligible subjects (n=501) to either one dose of placebo or inclisiran (200, 300, or 500 mg on day 1) or two doses of placebo or inclisiran (100, 200 or 300 mg on day 1 and 90) on top of standard care and stratified participants based on the presence (n=67) or absence (n=415) of diabetes at baseline.
This post-hoc analysis of the ORION-1 trial assessed whether individuals with and without diabetes at baseline respond differently to inclisiran with regard to changes in lipid profiles, safety, and glycemic control. The primary efficacy endpoint was the percent change from baseline LDL-c at day 180. Secondary efficacy endpoint included the percent change in lipid measure. Adverse events were documented up to day 210.
Main results
Inclisiran and LDL-c levels
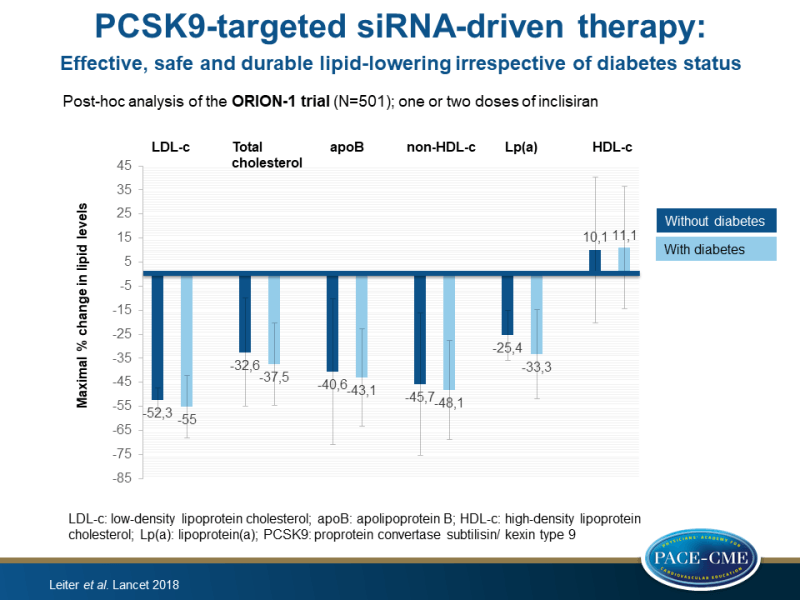
- The one-dose inclisiran regimen at the highest dose tested (500 mg) resulted in changes in LDL-c between baseline and the day 180 visit of -41.4% (95%CI: -47.0 to -35.9, P<0.0001) in those without diabetes and in -45.8% (95%CI: -62.3 to -29.3, P=0.0031) in those with diabetes, compared to placebo.
- Treatment with two-doses of inclisiran 300 mg resulted in robust reductions in mean LDL-c levels of 52.3% (95%CI: 57.1 to 47.5%, P<0.0001) in those without diabetes and of 55.0% (95%CI: 68.0 to 42.0, P<0.0001) in those with diabetes, from day 14 until day 180, compared to placebo.
- LDL-C remained significantly below baseline levels at day 180 with the one-dose inclisiran regimen.
- In the one-dose inclisiran regimen, the LDL-c nadirs normally occurred at day 30. The two-dose inclisiran regimen resulted in further LDL-lowering after the second dose.
Inclisiran and lipid profiles and glycemic control
- In both subjects without and with diabetes, treatment with inclisiran resulted in decreased total cholesterol (up to 32.6% [SD: 11.5] and up to 37.5 [8.8], respectively), atherogenic apoB (up to 40.6 [15.4] and up to 43.1 [10.3]), non-HDL-c (up to 45.7% [15.1] and up to 48.1 [10.5]), and Lp(a) (up to 25.4 [-37.4 to -15.0] and up to 33.3 [-51.7 to 15.9]), as well as in a trend towards increases in HDL-c (≤10.1% [15.5] and ≤11.1 [13.0]), compared to placebo.
- There were no clinically meaningful changes in HbA1c 180 days after treatment initiation, and this persisted over the course of the study.
Inclisiran and adverse events
- The number of adverse events was similar between patients with and without diabetes, and no inclisiran-related cases of myopathy, nor persistent elevations of liver function tests were seen.
Conclusion
Regardless of diabetes status and dose regimen, one or two doses of inclisiran on top of standard care not only yielded extended reduction of LDL-c levels, but this siRNA treatment also improved atherogenic lipid and lipoprotein profiles. Inclisiran treatment was found to be safe and well tolerated. These data suggest that inclisiran may be a viable lipid-lowering alternative in both people with and without diabetes.
References
1. Sabatine MS, Giugliano RP, Keech AC, et al.; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376:1713-1722
2. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:941-950
3. Ray KK, Colhoun HM, Szarek M, et al; ODYSSEY Outcomes Investigators. Alirocumab and cardiovascular outcomes in patients with acute coronary syndrome (ACS )and diabetes prespecified analyses of ODYSSEY OUTCOMES (Abstract). Diabetes 2018;67(Suppl.1):6-LB
4. Schwartz GG, Szarek M, Bhatt DL, et al. The ODYSSEY OUTCOMES trial: topline results alirocumab in patients after acute coronary syndrome. Presented at the 67th Scientific Sessions of the American College of Cardiology, 10– 12 March 2018, at the Orange County Convention Center, Orlando, FL
5. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk withelevatedLDLcholesterol.NEnglJMed2017; 376:1430-1440
6. Ray KK, Stoekenbroek RM, Kallend D, et al. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins. Circulation 2018; 138:1304-1316
