Lower VTE rate with DOAC in patients with cancer at risk for VTE, but more major bleeding
A randomized trial showed significantly lower risk of VTE with apixaban in ambulatory patients with cancer who were initiating chemotherapy with intermediate-to-high risk of VTE, compared to placebo, albeit with more major bleedings.
Apixaban to Prevent Venous Thromboembolism in Patients with CancerLiterature - Carrier M, Abou-Nassar K, Mallick R et al. - NEJM 2018; published online ahead of print
Introduction and methods
Patients with active cancer have an increased risk of venous thromboembolism (VTE), which leads to substantial morbidity and mortality [1-3]. The validated Khorana score may help to select patients with increased risk of VTE who could benefit from prophylaxis [4-7]. A small study has suggested that the use of the oral factor Xa inhibitor apixaban may be safe and effective in the prevention of VTE in patients with cancer [8]. Therefore, this trial aimed to establish the efficacy of apixaban thromboprophylaxis in ambulatory patients with cancer at risk for VTE.
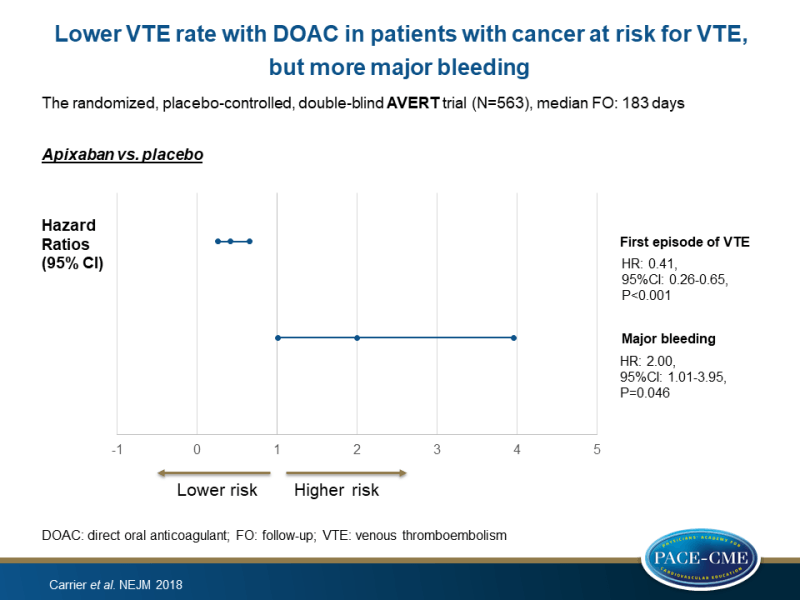
The placebo-controlled, double-blind AVERT trial (Feb 2014 – Apr 2018) randomized 563 patients (aged ≥18 years) with newly diagnosed cancer or progression of known cancer after complete or partial remission, who were at intermediate-to-high risk for VTE (Khorana score of ≥2), to either apixaban (2.5 mg daily) or placebo in a 1:1 ratio before administration of a first chemotherapy, and followed them for a median duration of 183 days. VTE was defined as any symptomatic or incidentally detected proximal deep-vein thrombosis of the lower or upper limbs, any non-fatal symptomatic or incidental pulmonary embolism, and pulmonary embolism–related death.
The primary efficacy outcome was the first episode of major VTE within the first 180 days after randomization. The main safety outcome was major bleeding.
Main results
Efficacy outcomes
- A first episode of VTE within 180 days was seen in 4.2% of patients treated with apixaban and in 10.2% of patients treated with placebo (HR: 0.41, 95%CI: 0.26-0.65, P<0.001).
- A competing-risk analysis that accounted for deaths from causes other than VTE or bleeding was consistent with the primary analysis (HR: 0.42, 95%CI: 0.27-0.65).
- The adjusted OR for VTE associated with the use of apixaban as compared with placebo was 0.39 (95%CI: 0.20-0.76).
- During the treatment period (median: 157 days), VTE occurred in 1.0% of the patients treated with apixaban and in 7.3% of those on placebo (HR: 0.14; 95%CI: 0.05-0.42).
- During the additional 30 days of follow-up after day 180, one patient in the apixaban group had deep-vein thrombosis and one in the placebo group died from pulmonary embolism.
Safety outcomes
- In the modified intention-to-treat analysis, major bleeding was observed in 3.5% of the patients treated with apixaban and in 1.8% of those treated with placebo (HR: 2.00, 95%CI: 1.01-3.95, P=0.046).
- 3 Of the 15 major bleeding episodes were considered to be a clinical emergency. No bleeding into critical organs was seen.
- During the treatment period, major bleeding was seen in 2.1% of the apixaban group and in 1.1% of the placebo group (HR: 1.89, 95%CI: 0.39-9.24).
- Adverse events were documented in 131 patients in the apixaban group and 127 patients in the placebo group; only one event in the apixaban group and two events in the placebo group were classified as being related to treatment with apixaban.
- Death from any cause occurred in 12.2% of the apixaban group and in 9.8% of the placebo group (HR: 1.29, 95%CI: 0.98-1.71). Of the 62 deaths, 87% were related to cancer or cancer progression.
Conclusion
A randomized trial showed significantly lower risk of VTE during treatment with apixaban in ambulatory patients with cancer who were initiating chemotherapy and who had an intermediate-to-high risk of VTE, compared to placebo, however, more major bleedings were observed.
References
1. Toft Sørensen H, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000; 343: 1846-50.
2. Blom JW, Vanderschoot JP, Oostindiër MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost 2006; 4: 529-35.
3. Otten HM, Mathijssen J, ten Cate H, et al. Symptomatic venous thromboembolism in cancer patients treated with chemotherapy: an underestimated phenomenon. Arch Intern Med 2004; 164: 190-4.
4. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008; 111: 4902-7.
5. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood 2010; 116: 5377-82.
6. van Es N, Di Nisio M, Cesarman G, et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study. Haematologica 2017; 102: 1494-501.
7. Khorana AA, Francis CW, Kuderer NM, et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thromb Res 2017; 151: 89-95.
8. Levine MN, Gu C, Liebman HA, et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost 2012; 10: 807-14.
