Treatment benefit of GLP-1RA in elderly with T2DM may vary with age
A posthoc analysis of the LEADER trial showed that liraglutide showed more pronounced CV benefits in T2DM patients aged 75 years or older, than in those between 60 and 74 years of age.
Effect of Liraglutide on Cardiovascular Outcomes in Elderly Patients: A Post Hoc Analysis of a Randomized Controlled TrialLiterature - Gilbert MP, Bain SC, Franek E et al., - Ann Intern Med. 2018. DOI: 10.7326/M18-1569
Introduction and methods
Comorbidities and complications associated with type 2 diabetes (T2DM) increase with age. Because this challenges treatment of elderly people with T2DM, the US Food and Drug Administration and European Medicines Agency recommend collecting comprehensive data on this patient group, to inform appropriate treatment of this growing group [1,2].
GLP-1 receptor agonists (GLP-1RA) have been demonstrated to give high glycemic efficacy and low intrinsic risk for hypoglycemia, and they also promote weight loss [3]. In the LEADER trial, the GLP-1RA liraglutide was shown to yield a 13% reduction in major adverse cardiovascular events (MACE), as compared with placebo, in patients with T2DM at high risk for CV events [4].
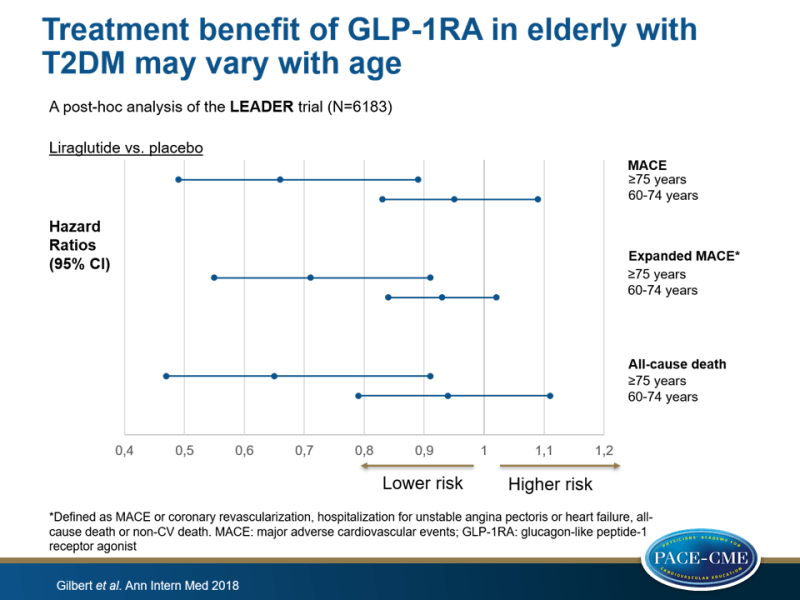
This posthoc analysis examined the CV effects of liraglutide vs. placebo in patients aged 75 years or older (n=836), and in those aged 60 to 74 years with risk factors for CV disease (n=6183). A secondary outcome was expanded MACE, defined as MACE or coronary revascularization, hospitalization for unstable angina pectoris or heart failure, all-cause death or non-CV death.
Main results
- The >75 group showed a 34% and 29% risk reduction in the frequency of MACE (HR: 0.66, 95%CI: 0.49-0.89) and expanded MACE (HR: 0.71, 95%CI: 0.55-0.91) with liraglutide vs. placebo.
- In those of 60-74 years old, the treatment effect of liraglutide was less pronounced (MACE: HR: 0.95, 95%CI: 0.83-1.09 and expanded MACE: HR: 0.93, 95%CI: 0.84-1.02).
- The P-value for interaction between age category and the effect of liraglutide on MACE was 0.054, and on expanded MACE was 0.084.
- All-cause death in patients >75 years was 35% lower with liraglutide vs. placebo (HR: 0.65, 95%CI: 0.47-0.91), as compared with 6% lower in those of 60-74 years (HR: 0.94, 95%CI: 0.79-1.11; P-interaction =0.088).
- Serious adverse events and nonserious medical events of special interest were reported in 63.5% and 61.7%, respectively. of patients >75, as compared with 49.5% and 49.8% in those between 60 and 74 years old. No notable differences were seen between treatment groups per age category.
- Liraglutide-treated patients experienced more gastrointestinal disorders (46.6% vs. 33.0%) and more acute gallstone disease (10.0% vs. 6.3%).
Conclusion
This post-hoc analysis of the LEADER trial showed that liraglutide significantly reduced the risk for MACE, expanded MACE and all-cause death in elderly patients at high risk for CV events, compared with placebo. The treatment benefit was more pronounced in patients older than 75 years, as compared to those aged 60 to 74 years.
References
1. U.S. Department of Health and Human Services; U.S. Food and Drug Administration; Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. Guidance for industry. E7 studies in support of special populations: geriatrics— questions and answers. 2012. Accessed at fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM189544.pdf on 18 September 2018.
2. Committee for Human Medicinal Products. Adequacy of guidance on the elderly regarding medicinal products for human use. European Medicines Agency. 2006. Accessed at ema.europa.eu/docs/en_GB/document_library /Scientific_guideline/2010/01/WC500049541.pdf on 18 September 2018.
3. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patientcentered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-9. [PMID: 25538310] doi:10.2337/dc14-2441
4. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-22. [PMID: 27295427] doi:10.1056/NEJMoa1603827
