Evaluation of DAPT duration in post-PCI patients stratified for bleeding score and ischemic risk
A retrospective analysis of RCTs suggests that when ischemic and bleeding risks are concordant, bleeding risk should inform decision-making on DAPT duration more so than ischemic risk.
Dual Antiplatelet Therapy Duration Based on Ischemic and Bleeding Risks After Coronary StentingLiterature - Costa F, Van Klaveren D, Feres F et al. - JACC 2019;73(7):741-54
Introduction and methods
The validated PRECISE-DAPT (PREdicting bleeding Complications in patients undergoing stent Implantation and SubsequEnt Dual AntiPlatelet Therapy) score can predict risk of bleeding in patients on dual antiplatelet therapy (DAPT) after stent implantation. Stratification of patients based on this score has been shown to be useful to inform decision-making for duration of DAPT in stented patients [1]. However, more research is needed on validation of this prediction tool in various subgroups, including subjects at higher ischemic risk.
A longer than average duration of DAPT is often prescribed in those with extensive coronary artery disease necessitating complex percutaneous revascularization techniques [2]. This is in accordance with findings from retrospective studies that patients on long-term DAPT after complex intervention have higher absolute ischemic risk reduction than those who received short-term DAPT [3,4]. On the other hand, complex intervention is linked with features that significantly increase bleeding risks, such as multi comorbidities, renal disease, or previous bleeding.
This retrospective analysis of RCTs therefore focused on the mutual role of ischemic and bleeding risks on outcomes. Moreover, the authors aimed to assess whether long- or short-term DAPT should be prioritized in the setting of patients with both high bleeding and ischemic risk features. The study included 14.963 patients treated with percutaneous coronary intervention (PCI) and subsequent DAPT. Patients treated with coronary stenting in an elective, urgent, or emergent setting were pooled at an individual level from 8 RCTs. In 5 out of 8 studies, DAPT duration was randomly assigned and two studies followed duration as recommended in guidelines and one based duration (1-12 months) on patient characteristics. Short-term DAPT was defined as 3 or 6 months and long-term as 12 or 24 months DAPT with aspirin and a P2Y12 inhibitor. Participants were further stratified based on ischemic and bleeding risks. High bleeding risk (HBR) was defined as PRECISE-DAPT score ≥25. Predicted ischemic risk after PCI was quantified based on criteria of PCI complexity. Complex PCI (n=3.118) was defined as occurrence of ≥1 complex PCI criterion, according to guidelines for PCI complexity [3,5].
The primary safety outcome consisted of TIMI major and minor bleeding. The primary efficacy outcome was a composite of myocardial infarction (MI), definite stent thrombosis (ST), stroke, or target vessel revascularization (TVR). Net adverse clinical events, which were obtained after pooling ischemic and bleeding events, as well as other ischemic and bleeding secondary endpoints, were also explored.
Main results
Clinical events with DAPT based on ischemic and bleeding risks
- The primary efficacy outcome was seen significantly more often in those with complex PCI, compared to those without complex PCI, both with (20.5% vs. 12.5%) and without (13.9% vs. 7.6%) HBR features.
- In the HBR group, major or minor bleeding events were roughly 3-fold higher, both with (5.8% vs. 1.8%, P<0.001) and without (4.8% vs. 1.4%, P<0.001) complex PCI features, compared to those without HBR.
- There was no significant association between fulfillment of complex PCI criteria alone and bleeding risk for TIMI major or TIMI minor bleeding.
Impact of randomized DAPT duration according to ischemic and bleeding risks
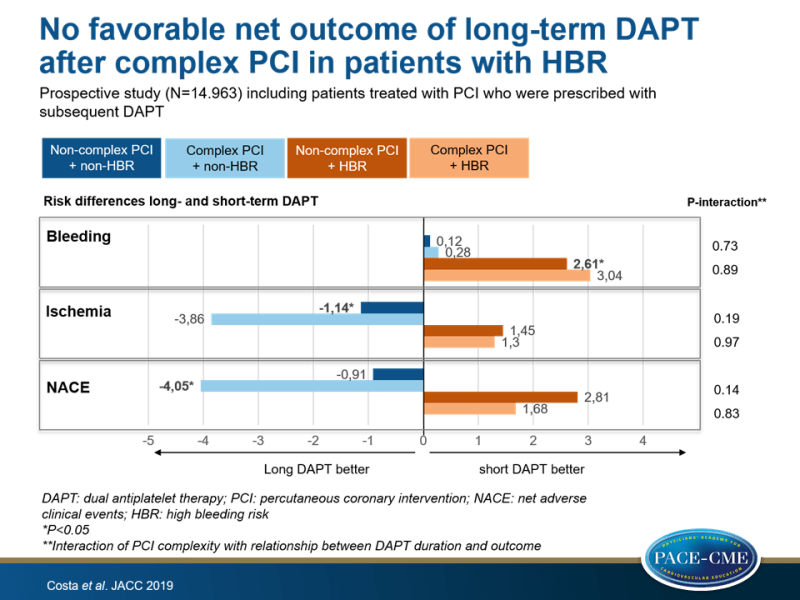
- In patients without HBR, long-term DAPT duration was associated with reduced ischemic events, with an absolute risk difference between long- vs. short-term DAPT in the non-complex PCI group of -1.14% (95%CI: -2.26% to -0.02%, P=0.04), and in the complex PCI group of -3.86% (95%CI: -7.71% to +0.06%, P=0.05)(P-interaction=0.19).
- Among those with HBR, long-term DAPT duration was not associated with a reduction of ischemic events, regardless of complex PCI features (non-complex PCI: +1.45% [95%CI: -1.84% to +4.72%] vs. complex: +1.30% [95%CI: -6.99% to +9.57%], P-interaction=0.97).
- In patients with HBR, long-term DAPT duration was associated with an excess of bleeding, regardless of PCI complexity, with a significant increase of TIMI major or minor bleeding among those treated with non-complex PCI (+2.61% [95%CI: +0.89% to +4.31%]) and a non-significant increase in the complex PCI group (+3.04% [95%CI: -2.97% to +8.82%], P-interaction=0.89).
- In those without HBR, long-term DAPT duration was not associated with higher bleeding risk, regardless of PCI complexity (non-complex PCI: +0.12% [95%CI: -0.25% to +0.50%] vs. complex: +0.28% [95%CI: -0.46% to +1.26%], P-interaction=0.73).
- When evaluating the net adverse clinical event endpoint by pooling ischemic and bleedings events, there was no significant net clinical benefit from long-term DAPT duration in patients without HBR, irrespective of PCI complexity.
- When stratifying for ACS presentation and/or procedural patient complexity, significant interactions were observed of patient complexity and the relation between treatment duration and ischemic and net adverse events, among those without HBR, but not in patients with HBR.
Conclusion
In this retrospective study of pooled data of RCTs, patients who underwent complex PCI had a higher risk for ischemic events compared with those with non-complex PCI. In patients without HBR, long-term DAPT duration was associated with reduced ischemic risk, especially in those with low PCI complexity. However, long-term DAPT did not result in additional benefit in patients with HBR features who underwent complex or non-complex PCI, which indicates an unfavorable net clinical outcome. These data suggest that when bleeding and ischemic risk are concordant, bleeding risk should inform decision-making on DAPT duration rather than ischemic risk.
Editorial comment
Angoulvant et al. [6] raise the question why long-term DAPT did not reduce ischemic events in the population of patients with high bleeding and ischemic risks. The investigators of this study also discussed this issue and hypothesized that other antithrombotic agents might be more efficient in reducing ischemic events. The higher bleeding risk population had higher number of patients for every component of the CHA₂DS₂-VASc score and it is suggested that CHA₂DS₂-VASc score predicts stroke in CAD patients. Therefore, Angoulvant et al. hypothesize that the lack of prevention of stroke could have had a substantial contribution to the observed findings of no reduction of total ischemic events with long-term DAPT in this subpopulation. Angoulvant et al. continue by questioning the robustness of the PRECISE-DAPT score since it was retrospectively derived and raise the need for prospective evaluation of bleeding risk scores in future clinical trials. They conclude by saying that this study is ‘a major signal for interventional cardiologists willing to individualize post-PCI DAPT duration’, but that the suggested strategy by Costa et al. needs to be tested in a prospective randomized clinical trial. Furthermore: ‘In future trials, investigators may want to investigate alternative strategies, such as DAPT versus low-dose aspirin, combined with a low-dose factor Xa inhibitor.’
References
1. Costa F, van Klaveren D, James S et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025–34.
2. Valgimigli M, Costa F, Byrne R et al. Dual antiplatelet therapy duration after coronary stenting in clinical practice: results of an EAPCI survey. Euro- Intervention 2015;11:68–74.
3. Giustino G, Chieffo A, Palmerini T et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol 2016;68:1851–64.
4. Yeh RW, Kereiakes DJ, Steg PG et al. Lesion complexity and outcomes of extended dual antiplatelet therapy after percutaneous coronary intervention. J Am Coll Cardiol 2017;70:2213–23.
5. Valgimigli M, Bueno H, Byrne RA et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–60.
6. Angoulvant D, Genet T, Ivanes F. Optimizing DAPT duration in high-risk patients after coronary stent implantation: bleeding risk takes it all. J Am Coll Cardiol. 2019;73:755-757.
