Better clinical outcomes with DOACs vs. warfarin in patients with NVAF and low body weight
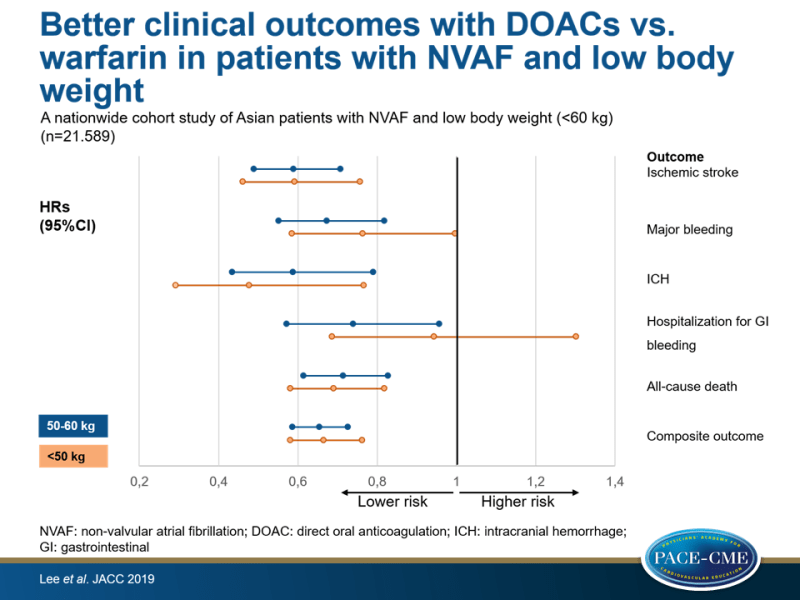
An Asian cohort of adults with non-valvular AF and low or very low body weight (<60/50 kg), showed better effectiveness and safety with regular and reduced DOAC dose, compared with warfarin.
Direct Oral Anticoagulants in Patients With Nonvalvular Atrial Fibrillation and Low Body WeightLiterature - Lee S-R, Choi E-K, Park CS et al. - JACC 2019;73(8):919-31
Introduction and methods
Direct oral anticoagulants (DOACs) are convenient, safe, and effective alternatives to warfarin for stroke prevention in patients with atrial fibrillation (AF) [1-3]. Effects of DOAC treatment are closely linked to plasma concentrations, which are dependent on body distribution volume. Extremely low body weight may thus affect the efficacy and safety of DOACs [4].
DOACs are associated with better net clinical benefit than warfarin, which is mainly a result of fewer intracranial hemorrhages (ICH). However, being underweight has been linked to an elevated risk of major bleeding in patients treated with DOACs. It remains unknown whether DOACs have similar beneficial effects in patients with low body weight, especially in those with extremely low body weight (<50 kg).
This nationwide cohort study therefore retrospectively compared the effectiveness and safety of DOACs with those of warfarin in Asian patients with non-valvular AF and low body weight. All patient data were obtained from the Korean National Health Insurance Service (NHIS) (n=50 million) and the National Health Insurance Corporation Health checkup database. Eligible patients (aged ≥20 years) had low body weight (≤60 kg) and newly prescribed warfarin (n=7.576) or DOAC (rivaroxaban, dabigatran, apixaban, or edoxaban, n=14.103) between January 2013 and December 2016, who did not have a history of ischemic stroke, ICH, or gastrointestinal (GI) bleeding. To balance the baseline characteristics between the warfarin and pooled DOAC groups, a weighting method based on propensity scores was used. Subanalyses were performed on for groups based on weight, DOAC dose and label adherence. Median follow-up was 1.2 years.
Outcomes were ischemic stroke, ICH, hospitalization for GI bleeding, hospitalization for major bleeding, all-cause death, and the composite of these outcomes.
Main results
Clinical outcomes in the total population with low body weight
- DOAC vs. warfarin use was associated with a significantly lower risk of ischemic stroke (HR: 0.59).
- DOAC treatment resulted in a significantly lower risk of major bleeding (HR: 0.71), which was mainly driven by a decrease in ICH (HR: 0.55), compared with warfarin. Risk of hospitalization for GI bleeding was reduced with DOAC treatment (HR: 0.82), compared with warfarin.
- There was an association between DOAC use and a significantly lower risk of all-cause death (HR: 0.71) and an improved composite outcome (HR: 0.66), compared with warfarin.
Clinical outcomes stratified by body weight
- Patients <50 kg were older, more likely to be women, had higher CHA₂DS₂-VASc score, and more often took a reduced DOAC dose (67% vs. 58%), compared to those weighing 50-60 kg.
- Compared to warfarin, DOAC use resulted in consistently lower incidence rates for ischemic stroke (2.50 vs. 3.56 per 100 person-years (PY) and 3.64 vs. 5.72 per 100 PY, for participants weighing 50-60 kg and in those <50 kg respectively), ICH (0.94 vs. 1.32 and 0.87 vs. 1.55), hospitalization for major bleeding (2.34 vs. 2.85 and 3.52 vs. 3.74), all-cause death (4.00 vs. 5.44 and 7.91 vs. 9.92), and for the composite outcome (7.96 vs. 10.5 and 13.0 vs. 16.8).
- In patients with body weight <50 kg, the DOAC group showed a lower incidence of hospitalization for major bleeding (3.52 vs. 3.74 per 100 PY) and improved composite outcome (13.0 vs. 16.8) despite comparable outcomes for hospitalization for GI bleeding (2.73 vs. 2.26) with DOAC use, compared to warfarin.
Clinical outcomes stratified by DOAC doses
- 61.9% Of DOAC users took a reduced dose. In propensity-score weighted analyses, patients with reduced DOAC dose had slightly higher incidence rates of ischemic stroke (3.16 vs. 2.77 per 100 PY) in the total population, similar to in patients of 50-60 kg and <50 kg.
- The incidence of ICH was slightly higher in those receiving a regular DOAC dose in the total population (0.96 vs. 0.90 per 100 PY) and in those weighing 50-60 kg (1.04 vs. 0.93 ), compared to those receiving a reduced DOAC dose, but not in patients <50 kg (0.74 vs. 0.83).
- In the <50 group, similar incidence rates were seen with a regular DOAC dose, compared with a reduced DOAC dose. However, wider CIs were observed due to the small size of the subgroups.
Conclusion
In this real-world Asian population with non-valvular AF and low body weight, DOAC use showed better effectiveness and safety compared with warfarin. This observation was consistent in patients with extremely low body weight (<50 kg), except that they showed more hospitalization for GI bleeding. Similar results were obtained in those receiving reduced doses of DOACs, compared to participants on regular DOAC dose, irrespective of body weight.
Editorial comment
In his editorial comment [5], Verheugt mainly focusses on DOAC dose in patients with AF. He explains that dose reductions are available on the drug labels for each DOAC. The therapeutic window of DOACs is small. He emphasizes: “A major problem is that inappropriate underdosing may lead to excess stroke and/or mortality, whereas overdosing can cause excess bleeding”.
In the study of Lee et al., inappropriate underdosing was seen most often with rivaroxaban (52%), followed by apixaban (35%), while this was not common with dabigatran and edoxaban. The dose reduction on the labels for apixaban and edoxaban is 50%, and that of dabigatran and rivaroxaban is 25%. Although these differences might have consequences for inappropriate dosing per DOAC, off-label underdosing did not result in worse outcomes in the current study. A recent review warned that DOACs might be linked to more bleeding and higher mortality in AF patients weighing <50 kg, but these novel observations mitigate such fears.
Next, Verheugt takes a closer look at the study design. A strength of the current study is the large number of patients with low body weight and that data are obtained from a national health database that are usually reliable and complete. However, he also emphasizes some limitations. First, the study originated from a registry, with its inherent shortcomings. Second, the investigators did not mention the INR and time in therapeutic range (TTR). In the current study, quality of warfarin management was lacking, ‘making the DOAC results an easy winner’. Third, the distribution of DOACs was not equal, with very little use of edoxaban (8% vs. 24% - 43% of the other DOACs). This makes the results of edoxaban versus warfarin questionable and the comparison between DOACs almost impossible. Finally, a great number of patients (62%) received reduced DOAC dose, whereas in the four pivotal DOAC trials, it varied from 5% with apixaban to 21% with rivaroxaban, 25% with edoxaban, and 50% (by design) with dabigatran. These data make it hard to compare with clinical practice, especially in the Western world. Only for edoxaban, a low body weight is the only criterion for dose reduction. For patients taking apixaban, there must be an additional feature for dose reduction: age >80 years and/or serum creatinine levels >1.5 mg/dL. In contrast, body weight does not play a role in prescription of dabigatran or rivaroxaban.
Verheugt concludes that dosing with both warfarin and DOACs is crucial, and that additional characteristics of patients with AF have to be taken into account in selecting patients that are eligible for DOAC dose reduction. “Although the current study is reassuring that DOACs can be used in AF patients with low to very low body weight, we must take into account that the study results originate from a region where low body weight is common. Because of this characteristic, the results do not necessarily apply for such patients in the Western world.”
References
1. Lee SR, Choi EK, Han KD et al. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int J Cardiol 2017;236:226–31.
2. Huisman MV, Rothman KJ, Paquette M et al. The changing landscape for stroke prevention in AF: finding from the GLORIA-AF registry phase 2. J Am Coll Cardiol 2017;69:777–85.
3. Ruff CT, Giugliano RP, Braunwald E et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62.
4. Park CS, Choi EK, Kim HM et al. Increased risk of major bleeding in underweight patients with atrial fibrillation who were prescribed non-vitamin K antagonist oral anticoagulants. Heart Rhythm 2017;14:501–7.
5. Verheugt FWA. Low Body Weight and Prescribing DOACs in Atrial Fibrillation. JACC 2019;73(8):932-4.
