No legacy effect with intensive glucose control for CV outcomes in T2DM after 15 years
In the follow-up study of VADT, original intensive glucose lowing over 5.6 years did not result in improved outcomes in T2DM patients vs. standard-therapy after 15 years, providing no evidence of a legacy effect.
Intensive Glucose Control in Patients with Type 2 Diabetes — 15-Year Follow-upLiterature - Reaven PD, Emanuele NV, Wiitala WL, et al. - N Eng J Med 2019;380:2215-24. DOI:10.1056/NEJMoa1806802
Introduction and methods
The ACCORD, ADVANCE, and VADT (Veterans Affairs Diabetes Trial) trials showed that improvement of glucose regulation over 3-6 years in type 2 diabetes (T2DM) patients did not result in reductions of CV events, nor reduction of CV mortality or total mortality [1-4]. Long term follow-up of original intensive glucose lowering in T2DM patients in these trials is needed to examine whether benefit with regard to CVD may emerge after a longer period, a so called ‘legacy effect’, as was found in the UKPDS [5]. Reduction in CV events was observed from the original intensive glucose lowering in T2DM patients after 10-year follow-up of the VADT [6], however, this was not evidence of a legacy effect as difference in HbA1c levels persisted between groups with intensive glucose regulation vs. standard and due to lack of statistical power.
With longer follow-up and good separation of the HbA1c curves between treatment groups during intervention, VADT follow-up (VADT-F) was a good study to examine long-term consequences of intensive glucose control on CV outcomes, quality of life (QoL) and mortality and to assess whether a legacy effect was present. In the VADT-F, a median of 5.6 years of intensive glucose lowering vs. standard lowering was followed by an observational period of nearly 10 years.
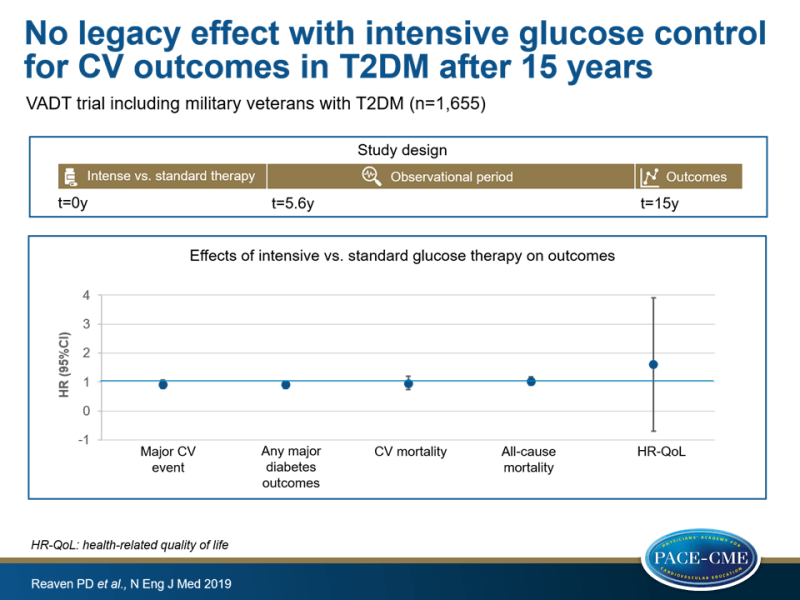
The VADT included 1791 military veterans with T2DM randomized to intensive vs. standard glucose control. HbA1c levels in the standard therapy group were between 8-9% and in the intensive-therapy group 1.5% lower than the standard-therapy group (6.9%). Difference in HbA1c was 0.2-0.3% 3 years after the trial ended, and levels finally stabilized at ~8% in both groups.
Participants in the VADT-F (n=1655) were followed using data from 4 national data registries, and for most participants (survey cohort, n=1391) yearly survey and chart reviews were done, and QoL was assessed. The prespecified primary outcome was first major CV event, a composite of nonfatal MI, nonfatal stroke, new or worsening congestive HF, amputation for ischemic gangrene, or CV death). Secondary outcomes were CV death, death from any cause, any major diabetes outcome, and health-related QoL. Median follow-up for primary-outcome events was 13.6 years, and for total mortality this was almost 15 years.
Main results
- Primary composite CVD outcome was not different between intensive-therapy vs. standard-therapy groups (HR: 0.91; 95%CI:0.78-1.06, P=0.23).
- Any major diabetes event (HR: 0.90, 95%CI:0.78-1.04) and CV death (HR: 0.94, 95%CI:0.73-1.20) were not different between groups.
- There was no benefit for mortality with intensive therapy vs. standard therapy (HR: 1.02, 95%CI:0.88-1.18)
- QoL scores were not different between groups.
- Comparing event rates of major outcomes during and after VADT showed no benefit for any major outcome during the observational follow-up period.
- Primary CV events were lower in the intensive-therapy group vs. the standard-therapy group during the ~10 year period when there was a separation of the HbA1c curves between the 2 groups (HR: 0.83, 95%CI: 0.70-0.99).
- In the 5-year period after the HbA1c curves were similar in both groups, there was no difference in risk of major CV events between groups (HR: 1.26, 95%CI:0.90-1.75).
- Adjustment for the cumulative mean HbA1c level during the entire study, or during the first 10-years abolished treatment-related differences in CV outcomes.
Conclusion
In this 15 year follow-up of the VADT, original intensive glucose control over a period of 5.6 years in T2DM patients did not result in benefits with regard to CV outcomes, total mortality or QoL when compared to standard-therapy. These results provide no evidence of a beneficial legacy effect for CV outcomes in T2DM patients after an original period of improved glucose control.
References
1. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545-59.
2. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129-39.
3 The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560-72.
4. Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009; 52: 2288-98.
5. Murray P, Chune GW, Raghavan VA. Legacy effects from DCCT and UKPDS: what they mean and implications for future diabetes trials. Curr Atheroscler Rep 2010; 12: 432-9.
6. Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372: 2197-206.
