Vitamin D supplementation not associated with reduced major adverse CV events
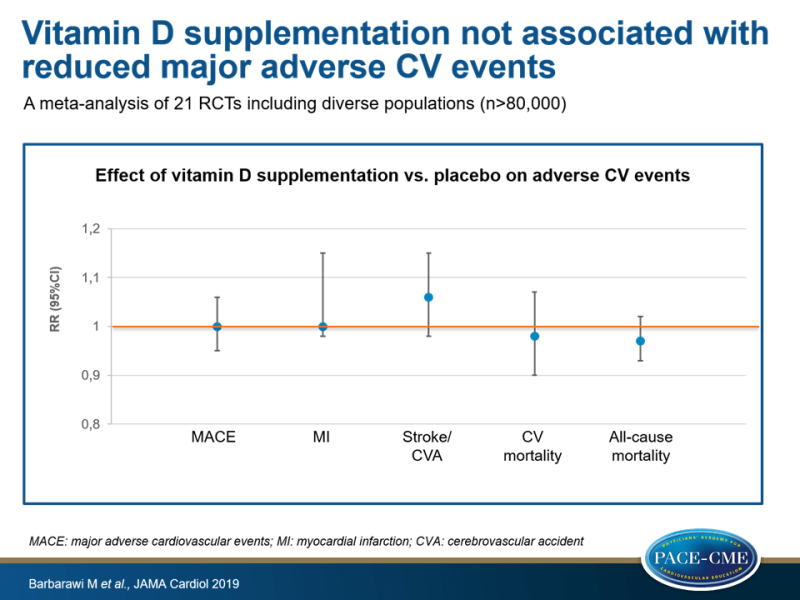
In a meta-analysis of 21 RCTs including diverse populations, supplementation with vitamin D did not result in a reduction of MACE, MI, stroke/CVA, CV mortality and all-cause mortality compared to placebo.
Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83000 Individuals in 21 Randomized Clinical Trials – A Meta-analysisLiterature - Barbarawi M, Kheiri B, Zayed Y, et al. - JAMA Cardiol 2019, doi:10.1001/jamacardio.2019.1870
Introduction and methods
Low serum vitamin D levels have been suggested to be associated with increased risk of CVD in observational studies [1-3]. Vascular tissues, such as the myocardium and vascular smooth muscle cells, express vitamin D receptors and vitamin D can thereby affect physiological functions [4,5]. In addition, vitamin D may have an effect on endothelial function and arterial thrombogenesis by influencing the renin-angiotensin-aldosterone system and parathyroid hormone [6-8].
In the US, the level of vitamin D supplementation has grown in primary care settings {9,10], but it is unknown whether vitamin D supplementation results in CV benefit. Therefore the US Preventive Services Task Force does not recommend vitamin D supplementation to prevent CVD [9]. In previous randomized clinical trials (RCTs), the association between vitamin D supplementation and CVD risk was examined with inconclusive data. Recent large-scale RCTs have added more evidence [11-14]. Therefore, a meta-analysis was performed to assess whether vitamin D supplementation can reduce CVD.
Embase, MEDLINE/Pubmed and Cochrane Library were searched for relevant RCTs from inception to December 2018. Eligibility criteria were long-term supplementation (≥1 year intervention) with vitamin D and report of CV outcomes. Any vitamin D or analogue supplementation was qualified. Primary endpoint was a composite of major adverse CV events (MACE). Secondary endpoints were MI, stroke/cerebrovascular accident (CVA), CVD mortality and all-cause mortality. 21 RCTs with 83291 participants were included in this analysis, 41669 received vitamin D supplementation and 41622 placebo. 8 trials included postmenopausal women, 9 trials older patients, 2 trials patients with chronic kidney disease, 2 trials with HF patients, and 1 trial patients with chronic obstructive pulmonary disease. Follow-up in the trials ranged from 1-12 years.
Main results
- Incidence of MACE was not different between those who took vitamin D and placebo (RR 1.0, 95%CI: 0.95-1.06, heterogeneity I²=11%).
- Subgroup analyses for age, sex, only postmenopausal women, pretreatment vitamin D levels <25 ng/mL, inclusion of patients with CKD, exclusion of trials with vitamin D analogues, vitamin D dosage and formulation did not result in significant findings.
- Compared to placebo, there was no association between vitamin D supplementation and MI (RR: 1.00, 95%CI: 0.98-1.15, I²=0%), stroke/CVA (RR: 1.06, 95%CI: 0.98-1.15, I²=0), CV mortality (RR: 0.98, 95%CI: 0.90-1.07, I²=2%) and all-cause mortality (RR: 0.97, 95%CI: 0.93-1.02, I²=0%).
Conclusion
In this meta-analysis of 21 RCTs including diverse populations (n> 80000 participants), supplementation with vitamin D did not result in reduction of MACE. In addition, risk of MI, stroke/CVA, CV mortality and all-cause mortality were not lowered in those who took vitamin D compared to placebo.
Editorial comment
In an editorial comment [15], Quyyumi and Al Mheid noted that > 80% of the participants in this meta-analysis participated in 3 recent large RCTs, the Vitamin D and Omega-3 Trial, the Vitamin D Assessment studies, and the Women’s Health Initiative. Other things to consider were that only 4 trials had CVD as primary endpoint, most trials were underpowered for CVD events, patient-level data was missing and the definition of MACE was trial-based. Furthermore, it is widely assumed that the association between vitamin D supplementation and outcomes is dose-dependent, but this is not likely. Also, selection and monitoring of study participants is not standard in supplementation and nutrient trials and inclusion of participants with sufficient vitamin D levels compromises the internal validity of trials examining the effect of vitamin D. However, subgroups with insufficient baseline vitamin D levels have not shown CV benefit of supplementation with vitamin D. It is also not ethical to withhold vitamin D in those with severe vitamin D deficiency because of established skeletal health benefits.
Quyyumi and Al Mheid end by stating that this study provides evidence that vitamin D testing and treatment should not be performed in populations not at risk for deficiency and not with the aim to prevent CVD morbidity and mortality. However, it should be noted that vitamin D is definitely indicated for patients with chronic kidney disease and hyperparathyroidism.
References
1. Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174-1180.
2. Pekkanen MP, Ukkola O, Hedberg P, et al. Serum 25-hydroxyvitamin D is associated with major cardiovascular risk factors and cardiac structure and function in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. 2015;25:471-478
3. Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414-1424.
4. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S-499S.
5. Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse.J Steroid Biochem Mol Biol. 2007;103:521-524.
6. Beveridge LA, Witham MD. Vitamin D and the cardiovascular system. Osteoporos Int. 2013;24: 2167-2180.
7. Al Mheid I, Patel RS, Tangpricha V, Quyyumi AA. Vitamin D and cardiovascular disease: is the evidence solid? Eur Heart J. 2013;34:3691-3698.
8. Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol. 2017;70:89-100.
9. LeFevre ML; U.S. Preventive Services Task Force. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015; 162:133-140.
10. Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999-2012.JAMA. 2016;316:1464-1474
11. Manson JE, Cook NR, Lee I-M, et al; VITAL Research Group. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44.
12. Shoji T, Inaba M, Fukagawa M, et al; J-DAVID Investigators. Effect of oral alfacalcidol on clinical outcomes in patients without secondary hyperparathyroidism receiving maintenance hemodialysis: the J-DAVID randomized clinical trial. JAMA. 2018;320:2325-2334.
13. Zittermann A, Ernst JB, Prokop S, et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J. 2017;38: 2279-2286.
14. Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study : a randomized clinical trial. JAMA Cardiol. 2017;2:608-616.
