NOAC alone as good as combination therapy in reducing ischemic outcomes in CAD and AF
ESC 2019 Rivaroxaban alone was non-inferior to combination therapy with regard to ischemic outcomes and superior with regard to bleeding in patients with CAD and AF.
Rivaroxaban Monotherapy versus Combination Therapy in Patients with Atrial Fibrillation and Stable Coronary Artery DiseaseNews - Sep. 2, 2019
Presented at the ESC congress 2019 in Paris, France by: Satoshi Yasuda (Suita, Japan)
Introduction and methods
After one year following revascularization in patients with stable CAD and AF or in patients not requiring intervention, guidelines recommend oral anticoagulant monotherapy. Evidence, however, from randomized controlled trials is lacking. In addition, in contrast to guideline recommendations, a high number of patients remain on combination therapy after one year.
Therefore, the AFIRE trial examined whether monotherapy with rivaroxaban is noninferior to combination therapy (rivaroxaban plus antiplatelet agent) in patients with AF and stable CAD, one year after revascularization or in patients not requiring revascularization. AFIRE was a multicenter, randomized, open-label, parallel-group trial, enrolling 2200 patients with AF and stable CAD, who were randomized to rivaroxaban monotherapy (10-15 mg/day) or combination therapy (rivaroxaban plus antiplatelet agent [aspirin, clopidogrel, prasugrel]).
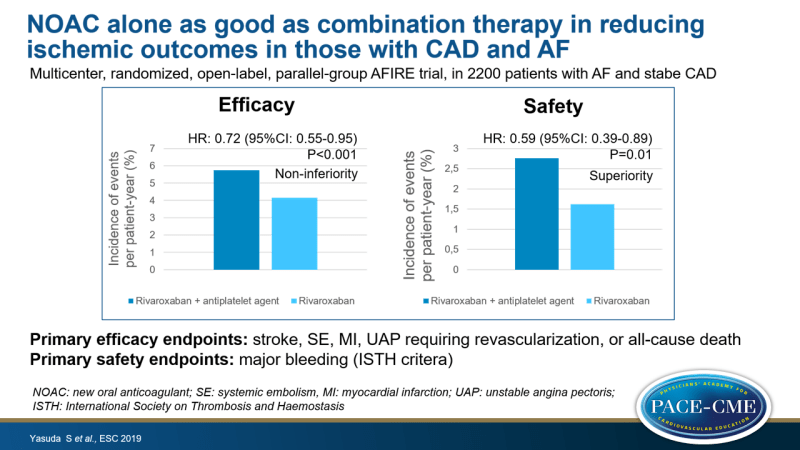
Primary efficacy endpoint was stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularization or death from any cause. Primary safety endpoint was major bleeding defined by ISTH criteria.
The trial was stopped early because of a higher risk of death from any cause in the combination therapy group. Median follow-up was two years.
Main results
- Primary efficacy events occurred at rate of 4.14% per patient-year in the monotherapy group and 5.75% per patient-year in the combination therapy group (HR: 0.72, 95%CI:0.55-0.95, Pnon-inferiority<0.001).
- Primary safety events occurred at rate of 1.62% per patient-year in the monotherapy group and 2.76% per patient-year in the combination therapy group (HR: 0.59, 95%CI:0.39-0.89, Psuperiority=0.01).
Conclusion
Monotherapy with rivaroxaban was non-inferior to combination therapy (rivaroxaban plus antiplatelet agent) with respect to schemic events and superior with respect to major bleeding. These findings support the recommendations that rivaroxaban should be used as monotherapy in patients with stable CAD and AF.
Discussion
In the discussion, it was asked whether type of antiplatelet agent might influence the outcome of bleeding. Yasuda said that 70% of patients were on aspirin and remaining on the P2Y12 inhibitor clopidogrel. The data have not been stratified for type of antiplatelet agent, and he could therefore not answer this question. In answer to the question on how high the risk of this population was, he gave the following median scores: CHADS₂: 2 CHA₂DS₂-VASc: 4 en HAS-BLED: 2.
In response to the question about generalizability and dosage, it was noted that the 50 mg dose used in Japan has similar effects as 20 mg used in whites.
Ruschitzka, one of the chairpersons of this session, said that this concept of monotherapy with a NOAC is a game changer in this field.
- Our reporting is based on the information provided at the ESC congress -