Genetically elevated Lp(a) levels associated with shorter parental life span
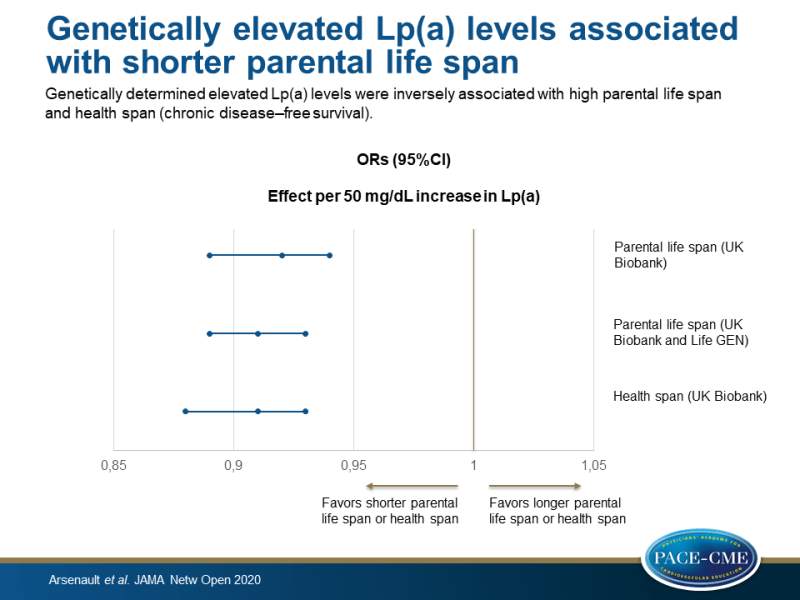
Genetically determined elevated Lp(a) levels were inversely associated with high parental life span and health span (chronic disease–free survival), and higher measured Lp(a) levels were associated with all-cause and CV mortality.
Association of Long-term Exposure to Elevated Lipoprotein(a) Levels With Parental Life Span, Chronic Disease–Free Survival, and Mortality Risk A Mendelian Randomization AnalysisLiterature - Arsenault BJ, Pelletier W, Kaiser Y et al., - JAMA Netw Open. 2020 ;3(2):e200129. doi: 10.1001/jamanetworkopen.2020.0129.
Introduction and methods
High levels of lipoprotein(a) (Lp(a)) are associated with a higher risk of atherosclerotic CVD [1-5]. However, results of studies that investigated the relationship between Lp(a) levels and Lp(a)-raising genetic variants with all-cause mortality are inconsistent. A study showed that one-quarter of healthy centenarian individuals had high Lp(a) levels in the absence of atherosclerotic CVD [6]. Another study found no association between high Lp(a) levels and all-cause mortality in patients with documented coronary heart disease [7]. However, a recent study in the general Danish population revealed an association between high Lp(a) levels and CV mortality and all-cause mortality [8]. Determining the association between high Lp(a) levels and longevity phenotypes could provide information on the potential of Lp(a)-lowering therapies to extend the life span in individuals with high Lp(a) levels.
This study used a 2-sample mendelian randomization (MR) design to determine whether genetic variants that are linked with elevated Lp(a) levels are associated with longevity phenotypes, estimated by parental life span and health span (age at the end of the chronic disease–free survival). The association between weighted genetic risk scores (wGRSs) of 26 independent single nucleotide polymorphisms (SNPs) at the LPA locus on parental life span was examined using individual participant data from the UK Biobank, as well as summary statistics of a genome-wide association study of more than 1 million life spans (joint analysis of the UK Biobank and LifeGen Consortium) [12]. For the MR individual data analysis, 139362 white individuals aged 55-69 years (mean age 62.8±3.9 years, 52% were women) from the UK Biobank [9] were enrolled. Participants were asked the age of their parents or the age at which their parents had died. wGRSs for Lp(a) levels were engineered using data from two previous studies [10,11]. Furthermore, summary statistics from a genome-wide association study on disease-free survival in the UK Biobank were used to study the association between LPA variants and health span [13].
This study also investigated the association between genetically determined and measured Lp(a) levels and long-term all-cause and CV mortality in the European Prospective Investigation Into Cancer and Nutrition (EPIC)-Norfolk study (18720 participants, 5686 mortality cases [2412 of CVD] during a mean follow-up of 20 years) [14].
Main results
- Genetically determined elevated Lp(a) levels were negatively associated with high parental life span (defined as at least 1 long-lived parent; father still alive and age >90 years or father’s age at death ≥90 years, or mother still alive and >93 years or mother’s age of death ≥93 years). The association per 50 mg/dL increase in Lp(a) with high parental life span had an odds ratio (OR) of 0.92 (95%CI 0.89-0.94, P=2.7×10^-8).
- A negative association was found between genetically determined Lp(a) levels and parental life span in the meta-analysis of the UK Biobank and LifeGen Consortium (effect per 50 mg/dL increase in Lp(a) on parental life span: OR 0.91, 95%CI 0.89-0.93, P<0.01)
- Genetically determined Lp(a) levels were negatively associated with health span in the UK Biobank (effect per 50 mg/dL increase in Lp(a) on health span: OR 0.91, 95%CI 0.88-0.93, P<0.01).
- Estimates of causal effects of Lp(a) levels on parental life span and health span were determined using inverse-variance-weighted (IVW)-MR and Egger-MR. A negative association was found between 26 LPA single-nucleotide polymorphisms (SNPs) and parental life span and health span (mean Egger-MR slope for parental life span: -0.0019 [SD:0.0002], P=2.22×10^-18, and for health span: -0.0019 [SD:0.0003], P=3.00×10^-13).
- Participants in the EPIC-Norfolk study with Lp(a) levels ≥50mg/dL had an increased risk for both all-cause and CV mortality compared to those with Lp(a) levels <50mg/dL (HR for all-cause mortality: 1.17, 95%CI 1.08-1.27, and HR for CV mortality: 1.54, 95%CI 1.37-1.72).
- The β coefficient for a comparison between high (≥95th percentile) vs low Lp(a) (<50th percentile) levels was 0.194 (SD:0.064). This corresponds to approximately 1.5 years in chronologic age for all-cause mortality risk, suggesting that the all-cause mortality risk for individuals with Lp(a) levels ≥95th percentile is equivalent to being 1.5 years older in chronologic age.
Conclusion
This study demonstrated that genetically determined elevated Lp(a) levels were negatively associated with high parental life span and health span. Individuals with Lp(a) levels ≥50mg/dL had a higher risk for all-cause and CV mortality, compared with those with Lp(a) levels <50mg/dL. These results give support for early identification and long-term treatment of individuals with high Lp(a) levels.
References
1. Zekavat SM, Ruotsalainen S, Handsaker RE, et al; NHLBI TOPMed LipidsWorking Group. Publisher correction:deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun. 2018;9(1):3493. doi:10.1038/s41467-018-05975-y
2. Emdin CA, Khera AV, Natarajan P, et al; CHARGE–Heart Failure Consortium; CARDIoGRAM Exome Consortium. Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J Am Coll Cardiol. 2016;68(25): 2761-2772. doi:10.1016/j.jacc.2016.10.033
3. Thanassoulis G, Campbell CY, Owens DS, et al; CHARGE Extracoronary CalciumWorking Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503-512. doi:10.1056/NEJMoa1109034
4. Arsenault BJ, Boekholdt SM, Dubé MP, et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis:a prospective mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7(3):304-310. doi:10.1161/CIRCGENETICS.113.000400
5. Erqou S, Kaptoge S, Perry PL, et al; Emerging Risk Factors Collaboration. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412-423. doi:10.1001/jama.2009.1063
6. Baggio G, Donazzan S, Monti D, et al. Lipoprotein(a) and lipoprotein profile in healthy centenarians: a reappraisal of vascular risk factors. FASEB J. 1998;12(6):433-437. doi:10.1096/fasebj.12.6.433
7. Zewinger S, Kleber ME, Tragante V, et al; GENIUS-CHD Consortium. Relations between lipoprotein(a) concentrations, LPA genetic variants, and the risk of mortality in patients with established coronary heart disease: a molecular and genetic association study. Lancet Diabetes Endocrinol. 2017;5(7):534-543. doi:10.1016/S2213-8587(17)30096-7
8. Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J. 2019;40(33):2760-2770. doi:10.1093/eurheartj/ehy902
9. Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi:10.1371/journal.pmed.1001779
10. Burgess S, Ference BA, Staley JR, et al; European Prospective Investigation Into Cancer and Nutrition–Cardiovascular Disease (EPIC-CVD) Consortium. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619-627. doi:10.1001/jamacardio.2018.1470
11. Mack S, Coassin S, Rueedi R, et al; KORA-Study Group. A genome-wide association meta-analysis on lipoprotein(a) concentrations adjusted for apolipoprotein(a) isoforms. J Lipid Res. 2017;58(9):1834-1844. doi:10.1194/jlr.M076232
12. Timmers PRHJ, Mounier N, Lall K, et al; eQTLGen Consortium. Genomics of 1 million parent life spans implicates novel pathways and common diseases and distinguishes survival chances. Elife. 2019;8:e39856. doi:10.7554/eLife.39856
13. Zenin A, Tsepilov Y, Sharapov S, et al. Identification of 12 genetic loci associated with human healthspan. Commun Biol. 2019;2:41. doi:10.1038/s42003-019-0290-0
14. Day N, Oakes S, Luben R, et al. EPIC-Norfolk: study design and characteristics of the cohort: European Prospective Investigation of Cancer. Br J Cancer. 1999;80(suppl 1):95-103.
