Addition of liraglutide reverses insulin-induced weight gain
Addition of a GLP-1 analogue to insulin therapy induced weight loss, while maintaining or even improving glycaemic control in T2DM patients with pronounced insulin-induced weight gain.
Liraglutide reverses pronounced insulin-associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT)Literature - de Wit HM et al., Diabetologia. 2014 - Diabetologia. 2014 Jun 20
de Wit HM, Vervoort GM, Jansen HJ et al.,
Diabetologia. 2014 Jun 20. [Epub ahead of print]
Background
Although insulin therapy is safe and efficacious in improving glycaemic control, it may induce weight gain. Interindividual differences in weight changes are large, even including weight loss in some [1].This undesirable effect occurs within the first 9-12 months of insulin therapy [1-3], thus a therapy that limits weight gain within this time frame is desirable.
Glucagon-like peptide-1 (GLP-1) analogues stimulate insulin secretion, suppress glucagon release, and reduce food intake, thereby improving glycaemic control and weight loss in type 2 diabetes (T2DM) [9-15]. Addition of GLP-1 analogues to insulin therapy may thus have advantages to reverse insulin-induced weight gain. It is, however, important to select the patients that will likely benefit most, due to the price and gastrointestinal side-effects of GLP-1 analogues. Long-term safety and efficacy data are not yet available and clinically relevant treatment strategies have not been sufficiently compared yet.
This study therefore compared addition of a GLP-1 analogue (liraglutide) to insulin therapy with the standard approach (continuation and intensification of insulin), in its ability to reverse pronounced insulin-induced weight gain, while maintaining glycaemic control in T2DM patients. 47 Patients were randomised to liraglutide or standard therapy. Most participants were obese (mean BMI: 33 kg/m2), with fair glucose regulation (mean HbA1c: 7.4%). On average, they had gained 7.1 kg while on insulin treatment.
Main results
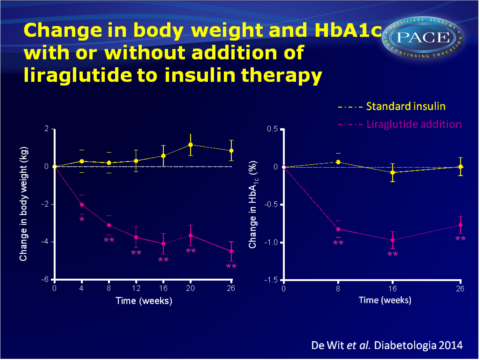
- After 26 weeks of treatment with liraglutide, body weight had decreased by 4.5+0.5 kg, while it had increased by 0.9+0.6 kg with standard therapy (maximum likelihood mean difference: -5.2, 95%CI: -6.7 to -3.6 kg, P<0.001), corresponding to a between-group difference in BMI of -1.7+0.3 kg/m2.
- HbA1c decreased by 0.77+0.11% with liraglutide, while it increased by 0.01+0.12% with standard therapy (maximum likelihood mean difference: -0.74%, 95%CI: -1.08% to -0.41%, P<0.001).
- 19 patients (73%) in the liraglutide group vs. 7 (29%) in the standard therapy group reached HbA1c > 7.0% (p<0.004).
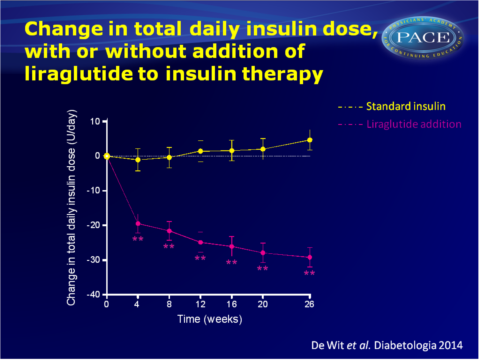
- Insulin dose was lowered by 29+3 U/day (-55.7+4.1%) with liraglutide, while it increased by 5+3 U/day (+11.8+4.4%) with insulin (maximum likelihood mean difference: -33u/day, 95%CI: -41 to -25 U/day, P<0.001).
- Adverse gastrointestinal events (decreased appetite, dyspepsia, constipation and nausea) were common, but mild-to-moderate in severity, and they often resolved after the first 4-8 weeks. At week 26, only 9 (35%) liraglutide-treated patients reported any gastrointestinal complaints. No serious adverse events were reported. 5 patients (19%) in the liraglutide group could discontinue insulin therapy.
- No changes in average total energy expenditure and physical activity, or quality of life were observed between the two treatment groups.
Download De Wit-ELEGANT diabetologia 2014 PACE.pptx
Conclusion
The results of the ELEGANT study show that addition of the GLP-1 analogue liraglutide to insulin, in fairly well-regulated patients with pronounced insulin-induced weight gain, yields reversal of weight gain, while continuation and intensification of insulin therapy led to further weight gain. Although it was not the aim of this study to alter glycaemic control, HbA1c decreased. Thus, glycaemic control was maintained or improved, while weight loss was induced.Adverse gastrointestinal events were mild-to-moderate and temporary for most patients, thus addition of liraglutide seems an attractive strategy to reverse weight gain in T2DM patients with pronounced insulin-induced weight gain.
Not all patients responded to therapy, but those who did, showed an effect within 12-16 weeks. Thus, a trial period of 3 months may be a suitable approach to test the efficacy of liraglutide in an individual patient.
Find this article on Pubmed
References
1. Jansen HJ, Hendriks JC, de Galan BE, et al. (2011) Contribution of change in glycosylated haemoglobin to insulin- associated weight gain: results of a longitudinal study in type 2 diabetic patients. Endocrine 39:190–197
2. Jansen HJ, Vervoort G, van der Graaf M, et al. (2010) Pronounced weight gain in insulin-treated patients with type 2 diabetes mellitus is associated with an unfavourable cardiometabolic risk profile. Neth J Med 68:359–366
3. Holman RR, Thorne KI, Farmer AJ et al (2007) Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 357:1716–1730
4. Salle A, Ryan M, Guilloteau G, et al. (2005) ‘Glucose control-related’ and ‘non-glucose control-related’ effects of insulin on weight gain in newly insulin-treated type 2 diabetic patients. Br J Nutr 94:931–937
5. Holst JJ, Deacon CF, Vilsboll T, et al. (2008) Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med 14:161–168
6. Marre M, Shaw J, Brandle M et al (2009) Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med 26:268–278
7. Nauck M, Frid A, Hermansen K et al (2009) Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combina- tion with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 32:84–90
8. Garber A, Henry R, Ratner R et al (2009) Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373:473–481
9. Zinman B, Gerich J, Buse JB et al (2009) Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 32:1224–1230
10. Russell-Jones D, Vaag A, Schmitz O et al (2009) Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 52:2046–2055
11. Nauck MA, Kemmeries G, Holst JJ, et al. (2011) Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 60:1561–1565.
