Amiodarone use together with warfarin increases risk of stroke/SE and lowers TTR
15/10/2014
Substudy of ARISTOTLE shows that apixaban consistently reduced the rate of stroke and SE, death and major bleeding when compared with warfarin, whether patients received amiodarone or not.
Amiodarone, Anticoagulation, and Clinical Events in Patients With Atrial Fibrillation: Insights From the ARISTOTLE TrialLiterature - Flaker G et al., J Am Coll Cardiol. 2014 - J Am Coll Cardiol. 2014 Oct 14
Flaker G, Lopes RD, Hylek E, et al; ARISTOTLE Committees and Investigators.
J Am Coll Cardiol. 2014 Oct 14;64(15):1541-50. doi: 10.1016/j.jacc.2014.07.967
Background
Amiodarone is an effective anti-arrhythmic drug for the prevention of atrial fibrillation (AF), recommended for patients with frequent, symptomatic AF episodes [1]. Amiodarone interferes with warfarin metabolism through the CYP 2C9 pathways, and makes it more difficult to maintain INR values of 2 to 3 [2-5]. This limits the use of amiodarone in patients with AF who receive warfarin to prevent stroke. Amiodarone may also cause noncardiovascular side-effects, including occasionally possibly life-threatening pulmonary toxicity [6,7]. Amiodarone has furthermore been associated with an increased risk of cancer [8-10] and excess non-CV deaths [11].
This study evaluated the effects of amiodarone on outcomes in the ARISTOTLE trial. The effects of apixaban or warfarin treatment on CV outcomes (stroke and systemic embolism (SE), death and bleeding) in relation to amiodarone use at the time of randomisation were compared. 2051 patients enrolled in ARISTOTLE received amiodarone and 15856 patients did not. Mean follow-up time was 21.7 months for the amiodarone group and 21.8 months for patient who were not on amiodarone.
Main results
- Patients assigned to warfarin showed a mean TTR of 56.5% when they also received amiodarone and 63.0% if they did not (P<0.0001). Mean time below range (INR<2) was 28.5% for those on amiodarone and 24.2% for those not on amiodarone (P<0.0001). 15% and 12.8% of time was spent above range (INR>3) in those with or without amiodarone respectively.
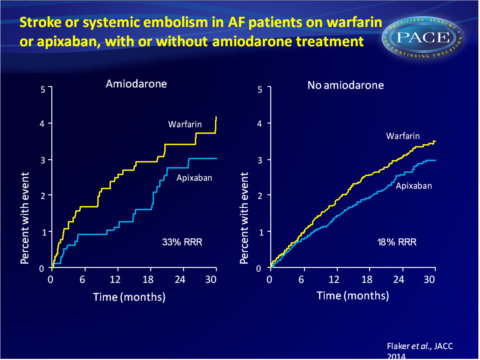
- For patients assigned to amiodarone, stroke/SE rate was 1.24%/year for those on apixaban and 1.85%/year for those assigned to warfarin (HR: 0.68, 95%CI: 0.40-1.145). In patients not assigned to amiodarone, the respective stroke/SE rates were 1.29% and 1.57%/year (HR: 0.82, 95%CI: 0.68-1.00).
- In amiodarone-receiving patients, the rate of all-cause death was 4.15%/year when assigned to apixaban and 5.65%/year when on warfarin (HR: 0.74, 95%CI: 0.55-0.98). In patients not on amiodarone, the rates were 3.43%/year on apixaban and 3.68%/year on warfarin (HR: 0.93, 0.83-1.05).
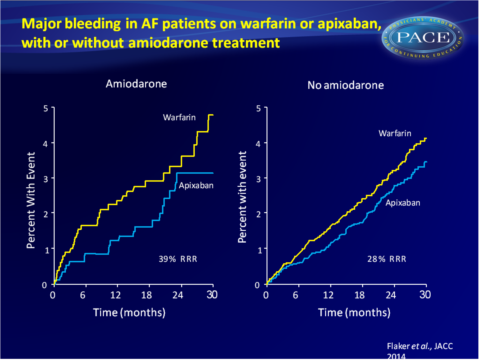
- The rate of major bleeding in patients receiving amiodarone was 1.86%/year on apixaban and 3.06%/year on warfarin (HR: 0.61, 95%CI: 0.39-0.96). In patients not on amiodarone at baseline, the rate was 2.18%/year on apixaban or 3.03%/year on warfarin (HR: 0.72, 95%CI: 0.62-0.84).
- A propensity score-adjusted analysis revealed that patients on amiodarone (1.58%/year) had a higher rate of stroke/SE than those not on amiodarone (1.19%/year, adjusted HR: 1.47, 95%CI: 1.03-2.10, P=0.0322). Amiodarone-treated patients showed numerically, but not statistically significantly higher rates of all-cause death, C death and non-CV death.
Download Flaker JACC 2014 PACE.pptx
Conclusion
This analysis of the ARISTOTLE trial shows that amiodarone use in patients with AF was associated with a significantly increased risk of stroke and SE and a lower TTR when used simultaneously with warfarin. Apixaban consistently and similarly reduced the rate of stroke and SE, death and major bleeding when compared with warfarin, whether patients received amiodarone or not.Find this article on Pubmed
References
1. Camm AJ, Lip GY, De Caterina R, et al. ESC Committee for Practice Guidelines. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2012;14:1385–413.2. Flaker GC, Pogue J, Yusuf S, et al., for the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE) Investigators. Cognitive function and anticoagulation control in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2010;3:277–83.
3. Kerin NZ, Blevins RD, Goldman L, et al. The incidence, magnitude, and time course of the amiodarone-warfarin interaction. Arch Intern Med 1988;148:1779–81.
4. Sanoski CA, Bauman JL. Clinical observations with the amiodarone/warfarin interaction: dosing relationships with long-term therapy. Chest 2002; 121:19–23.
5. Wallentin L, Yusuf S, Ezekowitz M, et al., for the RE-LY Investigators. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010;376:975–83.
6. Greenspon AJ, Kidwell GA, Hurley W, et al. Amiodarone-related postoperative adult respiratory distress syndrome. Circulation 1991;84:407–15.
7. Sobol SM, Rakita L. Pneumonitis and pulmonary fibrosis associated with amiodarone treatment: a possible complication of a new antiarrhythmic drug. Circulation 1982;65:819–24.
8. Piccini JP, Berger JS, O’Connor CM. Amiodarone for the prevention of sudden cardiac death: a meta-analysis of randomized controlled trials. Eur Heart J 2009;30:1245–53.
9. Su VY, Hu YW, Chou KT, et al. Amiodarone and the risk of cancer: a nationwide population-based study. Cancer 2013;119:1699–705.
10. Steinberg J, Sadaniantz A, Kron J, et al. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Circulation 2004; 109:1973–80.
11. Saksena S, Slee A, Waldo AL, et al. Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management): An assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses. J Am Coll Cardiol 2011;58:1975–85.
