Antisense inhibition of APOC3 lowers APOC3 and triglycerides in hypertriglyceridaemia
In a phase II study an APOC3 antisense oligonucleotide safely lowered APOC3, TGs and VLDL-c, and increased HDL-c and LDL-c, the latter of which was attenuated when ISIS 304801 was used as add-on to stable fibrate therapy.
Antisense Inhibition of Apolipoprotein C-III in Patients with HypertriglyceridemiaLiterature - Gaudet G et al., N Engl J Med 2015
Gaudet G, Alexander VJ, Baker BF et al.,
N Engl J Med 2015; 373:438-447 July 30, 2015 DOI: 10.1056/NEJMoa1400283
Background
Elevated triglyceride (TG) levels are associated with a wide range of pathological conditions, among which several relating to cardiovascular (CV) health. Patients with TG levels above 2000 mg/dL (22.6 mmol/L) are at high risk for pancreatitis [1,2]. Current guidelines recommend fasting TG levels below 1000 mg/dL (11.3 mmol/L) or 10 mmol/L [3,4], to prevent transient increases at which pancreatitis can develop. At moderate-to-high TG elevations, patients may be at risk of incident or recurrent coronary heart disease events.Apolipoprotein C-III (APOC3) is a central regulator of lipoprotein metabolism, with a key role in regulating plasma TG levels. APOC3 is a component of TG-rich lipoproteins, known to inhibit lipoprotein lipase-mediated hydrolysis of TG-rich lipoproteins and to negatively affect receptor-mediated hepatic uptake of remnants of TG-rich lipoproteins [5-10]. At higher concentrations, APOC3 inhibits hepatic lipase activity, which converts VLDL to IDL and LDL, and plays a role in the remodelling of HDL [11]. Thus, elevated APOC3 levels results in accumulation of atherogenic VLDL and chylomicron remnants, as a consequence of impaired lipolysis and disturbed clearance of TG-rich lipoproteins.
Elevated APOC3 levels, especially when present on apolipoprotein E-containing lipoproteins, are an independent risk factor for CVD. On the other hand, genetic variants causing lower APOC3 levels or function have been linked to lower risk of CHD. It remains to be determined whether targeted reduction of APOC3 will yield the same benefit in patients with high CV risk.
ISIS 304801 is an antisense oligonucleotide specifically binds to APOC3 mRNA and induces its degradation. Selective dose-dependent reduction of APOC3 protein and concomitant lowering of TG levels have been obtained with oligonucleotides in several preclinical animal models, and with ISIS 304801 in healthy volunteers [12], and in patients with the familial chylomicronaemia syndrome [13]. This randomised, placebo-controlled, dose-ranging phase 2 study evaluated the pharmacodynamics properties of 13 weeks ISIS 304801 as monotherapy and as add-on to stable doses of fibrate therapy in patients with severe or uncontrolled hypertriglyceridaemia.
Main results
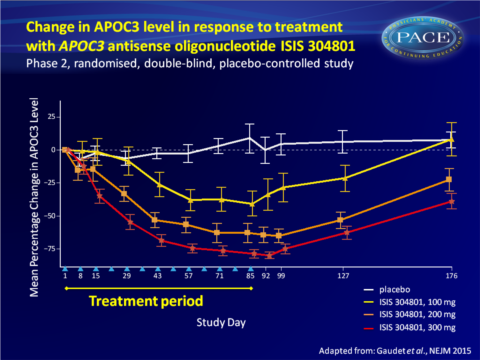
- Weekly subcutaneous injections of 100, 200 or 300 mg of ISIS 304801 produced a dose-dependent, prolonged reduction in plasma APOC3 levels from baseline.
- 300 mg ISIS 304801 yielded the largest percentage change from baseline (decrease of 79.6% vs increase of 4.2% with placebo, P<0.001).
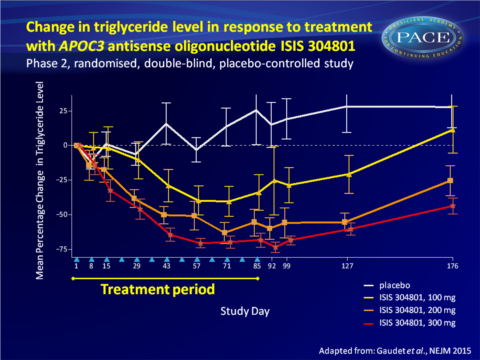
- A prolonged and dose-dependent decrease in TG levels was seen (largest decrease with 300 mg: 70.9% vs 20.1% increase on placebo), and HDL levels increased in a dose-dependent manner (300 mg: 45.7% vs. 0.7% with placebo).
- VLDL-c levels were also reduced in a dose-dependent manner (mean reduction from baseline 40.0%, 56.1% and 69.2% with 100, 200, and 300 mg respectively, vs increase of 6.3% with placebo), as were levels of VLDL APOC3 (-27.3%, -66.7% and -87.6%, vs. +21.0%).
- Dose-dependent increases in LDL-c were seen in ISIS 304801-treated groups (from overall mean at baseline of 79.5+29.9 mg/dL to 127.8+9 mg/dL). LDL apoB concentration also increased, as did LDL particle size. Non-HDL-c and total apoB remained relatively unchanged.
- When ISIS 304801 was given as add-on to fibrate therapy, 200 and 300 mg also showed dose-dependent and prolonged decreases in APOC3 levels (-70.9% with 300 mg vs. -2.2% with placebo).
VLDL-c and VLDL APOC3 levels were also substantially reduced with combination therapy. VDLD apoB decreased by 64.4% over the course of treatment with 300 mg. No changes were seen between the ISIS 304801-groups and placebo in levels of total cholesterol, non-HDL cholesterol, LDL-c and total apoB and LDL apoB. - Local cutaneous injection-site reactions were the most common adverse events, generally considered mild and transient. No treatment-associated safety issues were identified.
Download Gaudet NEJM 2015 PACE.pptx
Conclusion
This study demonstrates that inhibition of APOC3 synthesis with the second-generation antisense drug ISIS 304801 resulted in dose-dependent mean reductions of APOC3 (80%) and TG (71%) levels in patients with a broad range of baseline levels. ISIS 304801 as add-on to stable fibrate therapy yielded similar incremental reductions. The reductions in plasma APOC3 levels may have been the result of antisense oligonucleotide-induced decreases in hepatic APOC3 production, or of increased catabolism of TG-rich lipoproteins.While HDL-c increased and VLDL-c levels decreased, an increase in LDL-c and decrease in ApoB-48 were seen, all in a dose-dependent manner. The increase in LDL-c could potentially be offset by treatment with statins or fibrates. Indeed, the increase in LDL-c was less pronounced when ISIS 304801 was combined with fibrate therapy.
Find this article online at NEJM
References
1. Brunzell JD, Schrott HG. The interaction of familial and secondary causes of hypertriglyceridemia: role in pancreatitis. Trans Assoc Am Physicians 1973; 86: 245-54.
2. Brunzell JD, Schrott HG. The interaction of familial and secondary causes of hypertriglyceridemia: role in pancreatitis. J Clin Lipidol 2012; 6: 409-12.
3. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97: 2969-89.
4. Hegele RA, Ginsberg HN, Chapman MJ, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2: 655-66.
5. Aalto-Setälä K, Fisher EA, Chen X, et al. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice: diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest 1992; 90: 1889-900.
6. Windler E, Havel RJ. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res 1985; 26: 556- 65.
7. Sehayek E, Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Biol Chem 1991; 266: 18259-67.
8. Clavey V, Lestavel-Delattre S, Copin C, Bard JM, Fruchart JC. Modulation of lipoprotein B binding to the LDL receptor by exogenous lipids and apolipoproteins CI, CII, CIII, and E. Arterioscler Thromb Vasc Biol 1995; 15: 963-71.
9. Mann CJ, Troussard AA, Yen FT, et al. Inhibitory effects of specific apolipoprotein C-III isoforms on the binding of triglyceride- rich lipoproteins to the lipolysis- stimulated receptor. J Biol Chem 1997; 272: 31348-54.
10. Zheng C, Khoo C, Furtado J, Sacks FM. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation 2010; 121: 1722-34.
11. Brown RJ, Lagor WR, Sankaranaravanan S, et al. Impact of combined deficiency of hepatic lipase and endothelial lipase on the metabolism of both high-density lipoproteins and apolipoprotein B-containing lipoproteins. Circ Res 2010; 107: 357-64.
12. Graham MJ, Lee RG, Bell TA III, et al. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res 2013; 112: 1479-90.
13. Gaudet D, Brisson D, Tremblay K, et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med 2014; 371: 2200-6.
