Apixaban more effective than aspirin in reducing stroke recurrence
24/04/2012
During the International Stroke Conference 2012 results of a substudy of the AVERROES-trial (Apixaban Versus Acetylsalicylic Acid to Reduce the Risk of Stroke) were presented. These results are now published.
Apixaban more effective than aspirin in reducing stroke recurrenceLiterature - Diener HC, et al. Lancet Neurol 2012; 11: 225–31
Hans-Christoph Diener; John Eikelboom; Robert G Hart; Stuart J Connolly
Lancet Neurol 2012; 11: 225–31
A subgroup of patients was studied to compare apixaban in patients with (n = 764) and without (n = 4832) prior TIA or stroke.
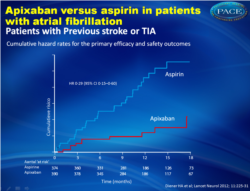
Only 10 patients (2.39% per year) with prior stroke and TIA who were randomized to apixaban had recurrent stroke or systemic embolism compared with 33 patients (9.16% per year) who were randomized to aspirin (HR = 0.29, 95 % CI, 0.15-0.60). Nine ischemic strokes occurred in the apixaban group (2.21% per year) versus 27 in the aspirin group (6.27% per year, HR = 0.33, 95% CI 0.15 to 0.69). Hemorrhagic stroke occurred in only 1 patient who received apixaban and in four who received aspirin.
In addition, mortality was higher with aspirin (7.9% vs. 5.8%, HR = 0.82, 95% CI, 0.47 to +1.45). There was no significant difference in major bleeding observed between the two groups.
Overall, these results translate to a number needed to treat (NNT) of 16 with apixaban versus aspirin in patients with stroke compared with an NNT of 74 in patients without prior stroke or TIA.
1. Eikelboom JW, O’Donnell M, Yusuf S, et al. Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J 2010; 159: 348–53 e1.
2. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364: 806–17.
Background In the AVERROES study, apixaban, a novel factor Xa inhibitor, reduced the risk of stroke or systemic embolism in patients with atrial fibrillation who were at high risk of stroke but unsuitable for vitamin K antagonist therapy. We aimed to investigate whether the subgroup of patients with previous stroke or transient ischaemic attack (TIA) would show a greater benefit from apixaban compared with aspirin than would patients without previous cerebrovascular events.
Methods In AVERROES, 5599 patients (mean age 70 years) with atrial fibrillation who were at increased risk of stroke and unsuitable for vitamin K antagonist therapy were randomly assigned to receive apixaban (5 mg twice daily) or aspirin (81–324 mg per day). The mean follow-up was 1•1 years. The primary efficacy outcome was stroke or systemic embolism; the primary safety outcome was major bleeding. Patients and investigators were masked to study treatment. In this prespecified subgroup analysis, we used Kaplan-Meier estimates of 1-year event risk and Cox proportional hazards regression models to compare the effects of apixaban in patients with and without previous stroke or TIA. AVERROES is registered at ClinicalTrials.gov, number NCT00496769.
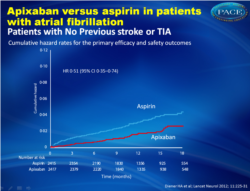
Findings In patients with previous stroke or TIA, ten events of stroke or systemic embolism occurred in the apixaban group (n=390, cumulative hazard 2•39% per year) compared with 33 in the aspirin group (n=374, 9•16% per year; hazard ratio [HR] 0•29, 95% CI 0•15–0•60). In those without previous stroke or TIA, 41 events occurred in the apixaban group (n=2417, 1•68% per year) compared with 80 in the aspirin group (n=2415, 3•06% per year; HR 0•51, 95% CI 0•35–0•74). The p value for interaction of the effects of aspirin and apixaban with previous cerebrovascular events was 0•17. Major bleeding was more frequent in patients with history of stroke or TIA than in patients without (HR 2•88, 95% CI 1•77–4•55) but risk of this event did not diff er between treatment groups.
Interpretation In patients with atrial fibrillation, apixaban is similarly effective whether or not patients have had a previous stroke or TIA. Given that those with previous stroke or TIA have a higher risk of stroke, the absolute benefits might be greater in these patients.
Lancet Neurol 2012; 11: 225–31
Background
During the International Stroke Conference 2012 results of a substudy of the AVERROES-trial (Apixaban Versus Acetylsalicylic Acid to Reduce the Risk of Stroke) were presented. These results are now published. In the AVERROES-trial 5599 patients with AF (mean age, 70 years) with an increased risk of stroke were included. Participants were randomized to apixaban 5 mg twice daily (Eliquis, Bristol-Myers Squibb / Pfizer) or aspirin 80 mg to 324 mg daily. Average follow-up was approximately 1 year [1,2].A subgroup of patients was studied to compare apixaban in patients with (n = 764) and without (n = 4832) prior TIA or stroke.
Main results
Only 10 patients (2.39% per year) with prior stroke and TIA who were randomized to apixaban had recurrent stroke or systemic embolism compared with 33 patients (9.16% per year) who were randomized to aspirin (HR = 0.29, 95 % CI, 0.15-0.60). Nine ischemic strokes occurred in the apixaban group (2.21% per year) versus 27 in the aspirin group (6.27% per year, HR = 0.33, 95% CI 0.15 to 0.69). Hemorrhagic stroke occurred in only 1 patient who received apixaban and in four who received aspirin.In addition, mortality was higher with aspirin (7.9% vs. 5.8%, HR = 0.82, 95% CI, 0.47 to +1.45). There was no significant difference in major bleeding observed between the two groups.
Conclusion
The oral factor Xa inhibitor apixaban was more effective than aspirin in reducing the risk of stroke and systemic embolism in patients with atrial fibrillation who have had a stroke or TIA previously and are unsuitable for warfarin therapy.
References
1. Eikelboom JW, O’Donnell M, Yusuf S, et al. Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J 2010; 159: 348–53 e1.2. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364: 806–17.
Abstract
Background In the AVERROES study, apixaban, a novel factor Xa inhibitor, reduced the risk of stroke or systemic embolism in patients with atrial fibrillation who were at high risk of stroke but unsuitable for vitamin K antagonist therapy. We aimed to investigate whether the subgroup of patients with previous stroke or transient ischaemic attack (TIA) would show a greater benefit from apixaban compared with aspirin than would patients without previous cerebrovascular events.Methods In AVERROES, 5599 patients (mean age 70 years) with atrial fibrillation who were at increased risk of stroke and unsuitable for vitamin K antagonist therapy were randomly assigned to receive apixaban (5 mg twice daily) or aspirin (81–324 mg per day). The mean follow-up was 1•1 years. The primary efficacy outcome was stroke or systemic embolism; the primary safety outcome was major bleeding. Patients and investigators were masked to study treatment. In this prespecified subgroup analysis, we used Kaplan-Meier estimates of 1-year event risk and Cox proportional hazards regression models to compare the effects of apixaban in patients with and without previous stroke or TIA. AVERROES is registered at ClinicalTrials.gov, number NCT00496769.
Findings In patients with previous stroke or TIA, ten events of stroke or systemic embolism occurred in the apixaban group (n=390, cumulative hazard 2•39% per year) compared with 33 in the aspirin group (n=374, 9•16% per year; hazard ratio [HR] 0•29, 95% CI 0•15–0•60). In those without previous stroke or TIA, 41 events occurred in the apixaban group (n=2417, 1•68% per year) compared with 80 in the aspirin group (n=2415, 3•06% per year; HR 0•51, 95% CI 0•35–0•74). The p value for interaction of the effects of aspirin and apixaban with previous cerebrovascular events was 0•17. Major bleeding was more frequent in patients with history of stroke or TIA than in patients without (HR 2•88, 95% CI 1•77–4•55) but risk of this event did not diff er between treatment groups.
Interpretation In patients with atrial fibrillation, apixaban is similarly effective whether or not patients have had a previous stroke or TIA. Given that those with previous stroke or TIA have a higher risk of stroke, the absolute benefits might be greater in these patients.