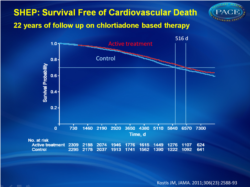
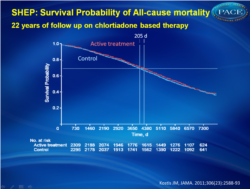
Association between chlorthalidone treatment of systolic hypertension and long-term survival.
04/01/2012
In the SHEP trial, treatment of isolated systolic hypertension with chlorthalidone stepped-care therapy for 4.5 years was associated with longer life expectancy at 22 years of follow-up.
Association between chlorthalidone treatment of systolic hypertension and long-term survival.Literature - Kostis JB et al, JAMA. 2011;306(23):2588-93
Kostis JB, Cabrera J, Cheng JQ, Cosgrove NM, Deng Y, Pressel SL, Davis BR.
JAMA. 2011;306(23):2588-93.
Abstract
Context In the Systolic Hypertension in the Elderly Program (SHEP) trial, conducted between 1985 and 1990, antihypertensive therapy with chlorthalidone-based stepped-care therapy resulted in a lower rate of cardiovascular events than placebo but effects on mortality were not significant.Objective
To study the gain in life expectancy of participants randomized to active therapy at the 22-year follow-up.Design,
Setting, and Participants A National Death Index ascertainment of death in the long-term follow-up of a randomized, placebo-controlled, clinical trial (SHEP) of patients aged 60 years or older with isolated systolic hypertension. Recruitment was between March 1, 1985, and January 15, 1988. After the end of a 4.5-year randomized phase of the SHEP trial, all participants were advised to receive active therapy. The time interval between the beginning of recruitment and the ascertainment of death by National Death Index (December 31, 2006) was approximately 22 years (21 years 10 months).
Main Outcome
Measures Cardiovascular death and all-cause mortality.
Results
At the 22-year follow-up, life expectancy gain, expressed as the area between active (n = 2365) and placebo (n = 2371) survival curves, was 105 days (95% CI, −39 to 242; P = .07) for all-cause mortality and 158 days (95% CI, 36-287; P = .009) for cardiovascular death. Each month of active treatment was therefore associated with approximately 1 day extension in life expectancy. The active treatment group had higher survival free from cardiovascular death vs the placebo group (hazard ratio [HR], 0.89; 95% CI, 0.80-0.99; P = .03) but similar survival for all-cause mortality (HR, 0.97; 95% CI, 0.90-1.04; P = .42). There were 1416 deaths (59.9%) in the active treatment group and 1435 deaths (60.5%) in the placebo group (log-rank P = .38, Wilcoxon P = .24). Cardiovascular death was lower in the active treatment group (669 deaths [28.3%]) vs the placebo group (735 deaths [31.0%]; log-rank P = .03, Wilcoxon P = .02). Time to 70th percentile survival was 0.56 years (95% CI, −0.14 to 1.23) longer in the active treatment group vs the placebo group (11.53 vs 10.98 years; P = .03) for all-cause mortality and 1.41 years (95% CI, 0.34-2.61; 17.81 vs 16.39 years; P = .01) for survival free from cardiovascular death.Conclusion In the SHEP trial, treatment of isolated systolic hypertension with chlorthalidone stepped-care therapy for 4.5 years was associated with longer life expectancy at 22 years of follow-up.
Background
The Systolic Hypertension in the Elderly Program (SHEP) was a randomized, placebo-controlled trial to assess the effect of antihypertensive treatment on the reduction of stroke in patients with isolated systolic hypertension [1]. From 1985 to 1990, patients enrolled in the trial were randomly assigned to active therapy (n=2365) or placebo (n=2371) for 4.5 years, after which all patients were assigned on active treatment.Over a period of 4.5 years, chlorthalidone-based therapy resulted in approximately 1 of 2 admissions for heart failure, 2 of 3 fatal or nonfatal strokes, and 1 of 4 coronary heart disease events [1-3].
Reduction of cardiovascular events was statistically significant, the effects on all-cause mortality and cardiovascular death were not.
At the end of the randomized phase of the trial, all participants received active treatment. This study investigated CV death and all-cause mortality of the SHEP trial 22 years after start of randomization.
In summary
Patients with isolated hypertension who were treated with a chlorthalidone-based stepped-care therapy for 4.5 years experienced a significantly lower rate of death and a longer life expectancy compared with placebo.References
1. SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265(24):3255-3264.2. Kostis JB, Davis BR, Cutler J, et al; SHEP Cooperative Research Group. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1997;278 (3):212-216.
3. Kostis JB, Berge KG, Davis BR, Hawkins CM, Probstfield J. Effect of atenolol and reserpine on selected events in the systolic hypertension in the elderly program (SHEP). Am J Hypertens. 1995; 8(12 pt 1):1147-1153.