Azilsartan gives better and persistent reduction in blood pressure than ramipril
29/03/2013
Better reduction in SBP and DBP obtained with 40 or 80 mg AZL-M than with 10 mg RAM, without increasing adverse effects.
Antihypertensive efficacy of the angiotensin receptor blocker azilsartan medoxomil compared with the angiotensin-converting enzyme inhibitor ramipril.Literature - Bönner G, Bakris GL, Sica D et al - J Hum Hypertens. 2013 Mar 21. doi: 10.1038/jhh.2013.6.
Bönner G, Bakris GL, Sica D et al.
J Hum Hypertens. 2013 Mar 21. doi: 10.1038/jhh.2013.6.
When uncontrolled, hypertension is associated with increased risk of several cardiovascular diseases. International guidelines for treatment of hypertension recommend a target blood pressure (BP) of <140/90 mmHg [1-4], but hypertension remains inadequately controlled in many patients [5]. Many effective antihypertensive drugs are associated with dose-limiting side effects that prevent their use at a high dose that may be necessary for optimal BP reduction. Angiotensin-converting enzyme (ACE) inhibitors are very effective but often come with significant cough and more rarely with angiooedema [6]. More potent and well-tolerated antihypertensive agents are needed. Angiotensin II-receptor blockers (ARBs) might offer the needed potency and tolerance.
Azilsartan medoxomil (AZL-M) is a prodrug that is rapidly hydrolyzed to azilsartan during absorption in the gastrointestinal tract. Azilsartan has high affinity for angiotensin II type 1 receptor.
The present randomised, double-blind, multicenter study compares the efficacy, safety and tolerability of once-daily (QD) AZM-L 40 and 80 mg with QD ramipril (RAM) 10 mg, which is the most commonly used dose strength and the highest dose approved in Europe. 884 patients with stage 1 or 2 hypertension received 24 weeks of treatment, after having discontinued their previous medication.
Both doses of AZL-M were superior to RAM in quickly and durably reducing trough, clinic and ambulatory SBP and DBP. There were no apparent differences between the 40 and 80 mg doses. Consistently greater BP control and response rates were obtained with AZL-M treatment. The safety profile of AZL-M was comparable with that of RAM, with less cough reported after AZL-M but slightly more dizziness and hypotension, likely due to the stronger BP reduction. The favourable efficacy and safety profile of AZL-M could be valuable for better persistence during chronic therapy and more patients achieving BP control.
2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Grenn LA, Izzo JL et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA 2003; 289(19):
2560–2572.
3. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G et al. Guidelines for the Management of Arterial Hypertension. J Hypertens 2007; 25(6): 1105–1187.
4. Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. Blood Press 2009; 18(6): 308–347.
5. Kotseva K, Wood D, De Backer G, De Bacquer D, Pyo‥ ra‥ la‥ K, Keil U. for the EUROASPIRE Study Group. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European
countries. Eur J Cardiovasc Prev Rehabil 2009; 16(2): 121–137.
6. Graettinger WF, 2003Systemic Hypertension. In: Crawford MH (eds). Current Diagnosis and Treatment in Cardiology. 2nd edn. (MacGraw-Hill Professional: New York, NY, USA, pp 167–178.
See abstract on Pubmed
J Hum Hypertens. 2013 Mar 21. doi: 10.1038/jhh.2013.6.
Background
When uncontrolled, hypertension is associated with increased risk of several cardiovascular diseases. International guidelines for treatment of hypertension recommend a target blood pressure (BP) of <140/90 mmHg [1-4], but hypertension remains inadequately controlled in many patients [5]. Many effective antihypertensive drugs are associated with dose-limiting side effects that prevent their use at a high dose that may be necessary for optimal BP reduction. Angiotensin-converting enzyme (ACE) inhibitors are very effective but often come with significant cough and more rarely with angiooedema [6]. More potent and well-tolerated antihypertensive agents are needed. Angiotensin II-receptor blockers (ARBs) might offer the needed potency and tolerance.Azilsartan medoxomil (AZL-M) is a prodrug that is rapidly hydrolyzed to azilsartan during absorption in the gastrointestinal tract. Azilsartan has high affinity for angiotensin II type 1 receptor.
The present randomised, double-blind, multicenter study compares the efficacy, safety and tolerability of once-daily (QD) AZM-L 40 and 80 mg with QD ramipril (RAM) 10 mg, which is the most commonly used dose strength and the highest dose approved in Europe. 884 patients with stage 1 or 2 hypertension received 24 weeks of treatment, after having discontinued their previous medication.
Main results
- After 24 weeks of treatment, trough, sitting, clinic systolic BP (SBP) had decreased significantly in all groups, but changes from baseline were significantly greater in the AZL-M 40 and 80 mg group (-20.6 +/- 0.95 and -21.2 +/- 0.95 mmHg respectively) as compared to RAM 10 mg (-12.2 +/- 0.95 mmHg)(P<0.001 for both comparisons).
- Changes in trough, sitting diastolic BP (DBP) was -10.2 +/- 0.55 mmHg in the AZL-M 40, and -10.5 +/- 0.55 mmHg in the AZL-M 80 group, as compared to -4.9 +/- 0.56 mmHg in the RAM 10 mg group (P<0.001 for both comparisons).
- The largest reduction in SBP and DBP was obtained by week 4 (after only 2 weeks at the highest dose), in each treatment arm, and was maintained until week 24.
- Ambulatory SBP and DBP were also reduced significantly more after AZL-M treatment (40 and 80 mg), as compared to RAM treatment, in all time intervals evaluated.
- The AZL-M 40 and RAM 10 group showed a similar incidence of adverse events, while they were slightly higher in de AZL-M 80 group. The RAM group reported higher rates of cough, whereas the AZL-M groups higher rates of dizziness and hypotension. Discontinuation due to adverse events were less frequent with AZL-M 40 (2.4%) and 80 mg (3.1%) as opposed with RAM (4.8%).
Conclusion
Both doses of AZL-M were superior to RAM in quickly and durably reducing trough, clinic and ambulatory SBP and DBP. There were no apparent differences between the 40 and 80 mg doses. Consistently greater BP control and response rates were obtained with AZL-M treatment. The safety profile of AZL-M was comparable with that of RAM, with less cough reported after AZL-M but slightly more dizziness and hypotension, likely due to the stronger BP reduction. The favourable efficacy and safety profile of AZL-M could be valuable for better persistence during chronic therapy and more patients achieving BP control.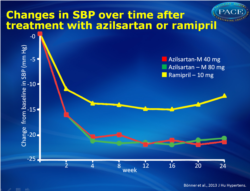 Figure 1Changes in clinic SBP and over time. The differences between the two AZL-M groups and the RAM group at week 24 were highly significant for both SBP and DBP (Po0.001). |
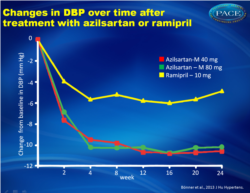 Figure 2Changes in clinic SBP and over time. The differences between the two AZL-M groups and the RAM group at week 24 were highly significant for both SBP and DBP (Po0.001). |
References
1.Whitworth JA. World Health Organization, International Society of Hypertension Writing Group. World Health organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21(11): 1983–1992.2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Grenn LA, Izzo JL et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA 2003; 289(19):
2560–2572.
3. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G et al. Guidelines for the Management of Arterial Hypertension. J Hypertens 2007; 25(6): 1105–1187.
4. Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. Blood Press 2009; 18(6): 308–347.
5. Kotseva K, Wood D, De Backer G, De Bacquer D, Pyo‥ ra‥ la‥ K, Keil U. for the EUROASPIRE Study Group. EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from 22 European
countries. Eur J Cardiovasc Prev Rehabil 2009; 16(2): 121–137.
6. Graettinger WF, 2003Systemic Hypertension. In: Crawford MH (eds). Current Diagnosis and Treatment in Cardiology. 2nd edn. (MacGraw-Hill Professional: New York, NY, USA, pp 167–178.
See abstract on Pubmed
