Azilsartan medoxomil/chlorthalidone for blood pressure reduction
03/07/2012
This study compared the antihypertensive efficacy, safety, and tolerability of azilsartan medoxomil plus chlorthalidone with olmesartan medoxomil plus hydrochlorothiazide in individuals with stage 2 systolic hypertension.
Azilsartan Medoxomil Plus Chlorthalidone Reduces Blood Pressure More Effectively Than Olmesartan Plus Hydrochlorothiazide in Stage 2 Systolic Hypertension.Literature - Cushman C et al.
Cushman WC, Bakris GL, White WB, Weber MA, Sica D, Roberts A, Lloyd E, Kupfer S.
Hypertension. 2012 Jun 18. [Epub ahead of print]
Background
Although the control of hypertension has improved over the last decade, in the US 31% of people treated for hypertension are not controlled to a blood pressure level <140/90 mm Hg [1]. There is a need for more effective antihypertensive regimens including fixed-dose combination products. Azilsartan medoxomil is a new, effective, long-acting angiotensin II receptor blocker (ARB). Compared with olmesartan and valsartan at maximal approved doses, azilsartan medoxomil has superior efficacy at its maximal dose, without increasing adverse events [2-4]. Combinations of azilsartan medoxomil with the potent, long-acting thiazide-like diuretic chlorthalidone are developed as an effective fixed dose combination.This study compared the antihypertensive efficacy, safety, and tolerability of azilsartan medoxomil plus chlorthalidone with olmesartan medoxomil plus hydrochlorothiazide in individuals with stage 2 systolic hypertension. Azilsartan medoxomil/chlorthalidone (AZL-M/CLD) was studied in doses of 40/25 and 80/25 mg versus olmesartan/hydrochlorothiazide (OLM/HCTZ) 40/25 mg. Clinic and ambulatory blood pressure measurements were used to assess efficacy. At baseline, mean clinic blood pressure was 165/96 mm Hg and 24-hour mean BP was 150/88 mm Hg.
Main results
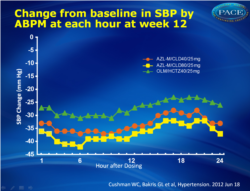
- Changes in clinic and ambulatory systolic blood pressures at week 12 were significantly greater in both azilsartan medoxomil/chlorthalidone arms than in the olmesartan/hydrochlorothiazide arm (P<0.001).
- Changes in clinic systolic blood pressure (mean±SE) were ±42.5±0.8, ±44.0±0.8, and ±37.1±0.8 mm Hg(AZL-M/CLD 40/25 mg, AZL-M/CLD 80/25 mg, and OLM/HCTZ 40/25 mg, respectively).
- Changes in 24-hour ambulatory systolic blood pressure were ±33.9±0.8, ±36.3±0.8, and ±27.5±0.8 mm Hg, respectively.
- Reports of serious events and events that led to discontinuation were similar for azilsartan medoxomil 40/25 mg and olmesartan/hydrochlorothiazide. There was a higher incidence of permanent discontinuation because of adverse events, but not serious adverse events, in the azilsartan medoxomil/chlorthalidone 80/25 mg group, primarily because of dizziness, serum creatinine increases, or hypotension.
Conclusion
The fixed-dose combination of azilsartan medoxomil/chlorthalidone provides a well-tolerated and more effective treatment for stage 2 systolic hypertension than olmesartan/hydrochlorothiazide.
References
1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050.2. White WB, Weber MA, Sica D, et al. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57:413–420.
3. Bakris GL, Sica D, Weber M, et al. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. J Clin Hypertens (Greenwich). 2011;13:81–88.
4. Sica D, White WB, Weber MA, et al. Comparison of the novel angiotensin II receptor blocker azilsartan medoxomil vs valsartan by ambulatory blood pressure monitoring. J Clin Hypertens (Greenwich). 2011;26:185–193.