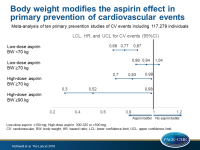
Body weight modifies the effect of aspirin in primary CV prevention
In primary prevention of CV events, low-dose aspirin was effective mainly in individuals weighing <70 kg, and higher doses of aspirin were of benefit for patients weighing ≥70 kg.
Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trialsLiterature - Rothwell PM, Cook NR, Gaziano JM, et al. - The Lancet 2018; published online ahead of print
Introduction and methods
Aspirin has been associated with lower long-term reductions in vascular events than expected based on its mode of action, possibly due to the use of too low doses in patients with higher body weight (BW) and vice versa [1]. In this meta-analysis of ten primary prevention studies events including 117,279 individuals [2-11], the modifying effects of BW on the effectiveness of low (≤100 mg) and higher (300-325 or ≥500 mg) doses of aspirin were evaluated. Moreover the long-term effect of aspirin on cancer risk is dependent on BW.
Primary prevention of vascular events trials were identified from the Antithrombotic Trialists (ATT)’ Collaboration, the Cochrane Collaboration Database of Systematic Reviews, and previous systematic reviews, and were considered eligible for the meta-analysis if they randomly assigned participants to aspirin versus no aspirin. Patient-level data were obtained from all eligible trials on baseline risk factors, major vascular events, and cancers. ATT Collaboration definitions of events [12] were used whenever possible. The results were validated using secondary prevention studies of stroke, comparing aspirin versus control or different aspirin doses with each other, if they had randomized at least 1,000 participants.
Main results
- For participants on low-dose aspirin weighing <70 kg, the odds ratio of CV events was 0.77 (419 vs 537 CV events; 95%CI: 0.68–0.87; P<0.0001; P-heterogeneity=0.32), and for participants weighing ≥70 kg it was 0.94 (791 vs 840 CV events; 95%CI: 0.86–1.04; P=0.24; P-heterogeneity=0.50).
- Low-dose aspirin reduced CV events to a lesser extent with increasing BW (P-interaction=0.0072), with the best effect occurring in participants weighing 50–69 kg (HR: 0.75; 95%CI: 0.65–0.85; P<0.0001), particularly with daily use (HR: 0.68; 95%CI: 0.56-0.83; P=0.0001).
- In participants weighing ≥70 kg, low-dose aspirin was associated with an increase in case fatality of first CV events (OR: 1.33; 95%CI: 1.08–1.64; P=0.0082).
- The increased risk of major bleeding on low-dose aspirin versus control was lost in participants weighing ≥90 kg (P-interaction=0.024).
- Higher doses of aspirin reduced the risk of CV events to a greater extend with increasing BW (HR for 325 mg aspirin in participants weighing ≥70 kg: 0.83; 95%CI: 0.70–0.98; P=0.028; HR for 500 mg aspirin in participants weighing ≥90 kg: 0.52; 95%CI: 0.30–0.89; P=0.017).
- The results were similar in men and women, those with diabetes, and in secondary prevention of stroke studies (P-interaction=0.0025 for CV events).
- Low-dose aspirin was associated with a reduced risk of colorectal cancer in participants weighing <70 kg (HR: 0.64; 95%CI: 0.50–0.82; P=0.0004), but not in those weighing ≥70 kg (HR: 0.87; 95%CI: 0.71–1.07; P=0.32), whereas higher doses of aspirin were of benefit for participants weighing up to 80 kg (HR: 0.69; 95%CI: 0.55–0.87; P=0.0014).
Conclusion
BW modifies the effect of aspirin in primary prevention of CV events. Low-dose aspirin was more effective in individuals weighing <70 kg, while higher doses of aspirin were of benefit for patients weighing ≥70 kg. These findings suggest that aspirin doses should be individualized in the setting of primary CV prevention. BW also affected the effect of aspirin on the risk of colorectal cancer in patients up to 80 kg.
References
1. Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2016;164: 836–45.
2. Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. BMJ 1988; 296: 313–16.
3. Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med 1989; 321: 129–35.
4. Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet 1998; 351: 233–41.
5. Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352: 1293–304.
6. Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337: a1840.
7. Fowkes FG, Price JF, Stewart MC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA 2010;303: 841–48.
8. Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351: 1755–62.
9. Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet 2001; 357: 89–95.
10. Ogawa H, Nakayama M, Morimoto T, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300: 2134–41.
11. Ikeda Y, Shimada K, Teramoto T, et al. Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014; 312: 2510–20.
12. Antithrombotic Trialists Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324: 71–86.