Canakinumab Anti-inflammatory Thrombosis Outcomes Study
22/12/2011
Inflammation contributes to all phases of the atherothrombotic process, and patients with elevated inflammatory biomarkers such as hCRP have increased vascular risk. Yet, it remains unknown whether direct inhibition of inflammation will reduce cv event rates..
Interleukin-1β inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS).Literature - Ridker et al, Am Heart J. 2011 Oct;162(4):597-605.
Paul M Ridker, MD, Tom Thuren, MD, Andrew Zalewski, MD, and Peter Libby, MD
Am Heart J. 2011 Oct;162(4):597-605.
Background
Inflammation contributes to all phases of the atherothrombotic process, and patients with elevated inflammatory biomarkers such as high-sensitivity C-reactive protein (hsCRP) have increased vascular risk. Yet, it remains unknown whether direct inhibition of inflammation will reduce cardiovascular event rates.Design
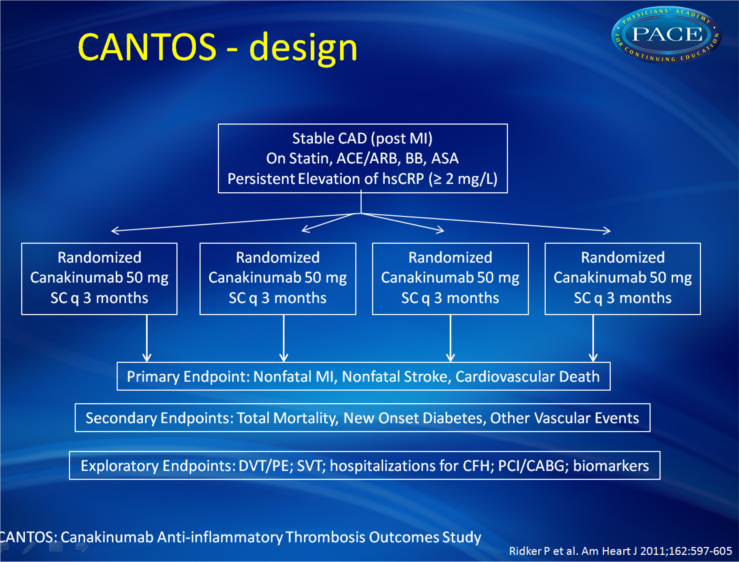
The CANTOS will evaluate whether interleukin-1β (IL-1β) inhibition as compared with placebo can reduce rates of recurrent myocardial infarction, stroke, and cardiovascular death among stable patients with coronary artery disease who remain at high vascular risk due to persistent elevations of hsCRP (>2 mg/L) despite contemporary secondary prevention strategies. Canakinumab is a human monoclonal antibody that selectively neutralizes IL-1β, a proinflammatory cytokine that plays multiple roles in the atherothrombotic process and that undergoes activation by the nucleotide-binding leucine-rich repeat-containing pyrin receptor 3 inflammasome, a process promoted by cholesterol crystals. Canakinumab significantly reduces systemic C-reactive protein and other inflammatory biomarker levels, is generally well tolerated, and is currently indicated for the treatment of inherited IL-1β driven inflammatory diseases such as the Muckle-Wells syndrome. In a multinational collaborative effort using an event-driven intention-to-treat protocol, CANTOS will randomly allocate 17,200 stable postmyocardial infarction patients with persistent elevation of hsCRP to either placebo or to canakinumab at doses of 50, 150, or 300 mg every 3 months, administered subcutaneously. All participants will be followed up over an estimated period of up to 4 years for the trial primary end point (nonfatal myocardial infarction, nonfatal stroke, cardiovascular death) as well as for other vascular events, total mortality, adverse events, and specific clinical end points associated with inflammation including new onset diabetes, venous thrombosis, and atrial fibrillation.Summary
If positive, CANTOS would confirm the inflammatory hypothesis of atherothrombosis and provide a novel cytokine-based therapy for the secondary prevention of cardiovascular disease and new-onset diabetes.Background
Inflammation plays an important role in atherothrombosis, mediated by several inflammatory mechanisms. The inflammatory biomarker high-sensitivity C-reactive protein (hsCRP) can be used to identify people at increased vascular risk [1]. Although it has been shown that hsCRP is largely associated with vascular risk [2], it is still unknown whether direct inhibition of inflammation will reduce vascular event rates [3]. One promising approach is inhibition of interleukin-1β (IL-1β), a potent proinflammatory cytokine that plays multiple roles in the atherothrombotic process.Canakinumab is a human monoclonal antihuman IL-1β antibody already indicated for IL-1β driven inflammatory diseases such as CAPS and Muckle-Wells syndrome. Canakinumab binds human IL-1β thereby blocking the interaction of this cytokine with its type I and II receptors. IL-1β antagonism with canakinumab results in a rapid and sustained inhibition of the acute phase response, leading to marked reductions in CRP and IL-6. Canakinumab has little effects on lipid levels, so that the inflammatory hypothesis of atherothrombosis by inhibiting inflammation can be tested without confounding co-effects. Due to the prolonged effect of canakinumab on the acute phase response, it can be dosed quarterly (SC). In the development program, canakinumab showed a favorable safety and tolerability profile.
The primary objective of the CANTOS is to determine whether long-term treatment with canakinumab (50/150/300 mg SC every 3 months) reduces rates of recurrent cardiovascular events compared to placebo in stable patients post-MI who remain at elevated vascular risk (hsCRP > 2mg/L) despite usual care, including statin treatment. Approximately 17,200 individuals (both sexes) of 18 years and older will be enrolled.
In summary,
CANTOS, if successful, can contribute to the inflammatory hypothesis of atherothrombosis and may provide a novel cytokine-based therapy for secondary prevention of cardiovascular disease and new-onset diabetes.References
1. Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007;49:2129-38.2. Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132-40.
3. Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J Thromb Haemost 2009;7(Suppl 1):332-9.