CETP inhibition increases HDL-c levels but only modestly improves HDL function
CETP inhibition by dalcetrapib increases HDL-c and apolipoprotein A1, as well as cholesterol efflux, mostly through non-ABCA1-mediated pathways.
The effect of cholesteryl ester transfer protein inhibition on lipids, lipoproteins, and markers of HDL function after an acute coronary syndrome: the dal-ACUTE randomized trialLiterature - Ray KK et al. Eur Heart J 2014 - Eur Heart J, 2014
Ray KK, Ditmarsch M, Kallend D, et al., on behalf of the dal-ACUTE Investigators
Eur Heart J (2014) doi: 10.1093/eurheartj/ehu105 online March 17, 2014
Prof. Kausik Ray, St George’s, University of London, about this study:
Our study looked at what HDL particles did and whether the CETP inhibition with dalcetrapib improved the function or efficiency of these particles. We found that HDL particles did increase in number and function but by a modest 10% despite raising HDL overall by 30%. So there is a discrepancy between cholesterol levels in good particles and how they are working and simply raising HDL, while having a significant effect on function, may not be enough. It is possible that the way that these particles work is more important than the overall levels of cholesterol they carry and maybe these types of drugs are only likely to be effective by increasing function much more.Importantly these data also suggest that more work needs to be done to see how function is related to heart attacks.Background
Although HDL-particles can have anti-atherosclerotic properties, measuring HDL-cholesterol (HDL-c) levels reflects the content of a variety of HDL particle subtypes with different shape, size and functions [1]. Based on observational data supporting a protective association between HDL-c and cardiovascular (CV) disease, raising HDL-c was explored as a therapeutic approach. Recent evidence suggests that not HDL-c levels per se, but specific HDL subclasses [2] and function, as assessed by cholesterol efflux capacity [3], are more important.The dal-OUTCOMES study showed that the cholesteryl ester transfer protein (CETP) inhibitor dalcetrapib raised HDL-c in patients with a recent acute coronary syndrome (ACS), but did not reduce CV events [4]. The data of the dal-OUTCOMES study suggest that HDL-c levels at baseline or on-treatment are not associated with clinical events. Instead, HDL particles may for instance be dysfunctional when produced in a pro-inflammatory milieu [5].
Due to clinical futility, the dalcetrapib study program was terminated, but the current study had been initiated before that decision. The dal-ACUTE study evaluates the short-to-medium-term effects of CETP inhibition with dalcetrapib on HDL-c, apolipoprotein A1, in vitro HDL efflux function and inflammatory biomarkers, in 300 patients, immediately after an ACS. Dal-ACUTE is a phase 3, double-blind, randomised and placebo-controlled study, with 24 weeks follow-up.
Main results
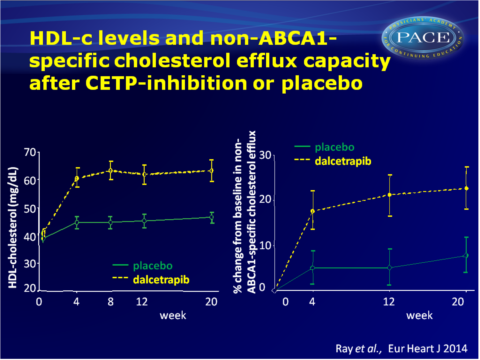
- At week 4, placebo-corrected increases in HDL-c and apolipoprotein A1 with dalcetrapib were 33.7 and 11.8% respectively. These increases were maintained throughout the study.
LDL-c and apolipoprotein B levels declined to a similar extent in placebo and dalcetrapib-treated patients, at any time point during the study.
Apolipoprotein E levels remained stable in the dalcetrapib group, while they decreased in the placebo group. - Pre-β1-HDL is the rate-limiting acceptor of cholesterol effluxed by the ATP-binding cassette transporter (ABC) A1 pathway. Pre-β1-HDL levels did not change significantly over time, nor between the two treatment groups.
- While in vitro ABCA1-specific cholesterol efflux was not significantly different over time and between treatments, it was significantly reduced in the highest quartile of high-sensitivity C-reactive protein (CRP) levels at baseline.
Dalcetrapib increased non-ABCA1-mediated efflux and total efflux by about 10% at 4, 12 and 20 weeks, and this effect seemed attenuated in those with high BMI, current smokers, and higher apolipoprotein B/A1 ratios at baseline. - Subtypes of HDL and lipoproteins were differentially correlated with various efflux pathways. Treatment with dalcetrapib did not change these correlations.
- After 4 weeks of treatment, apolipoprotein E was more strongly correlated with ABCA1-mediated efflux, but similarly in both treatment groups. The correlation between apolipoprotein E and non-ABCA1-mediated efflux was about 13-fold times stronger after 4 weeks as compared to baseline, only in the dalcetrapib group (R2: 0.38 vs. 0.03), and total efflux explained by apolipoprotein E was about 3-fold increased (R2: 0.38 vs. 0.13).
- Levels of CRP and HDL-associated serum amyloid A (HDL-SAA) decreased over time, to a similar extent in both treatment groups at week 4 and 20.
- Dalcetrapib was generally well tolerated; no significant differences in the number of adverse events were seen between treatment groups, with the exception of more positively adjudicated CV events observed in the dalcetrapib arm after 20 weeks.
Download Ray EHJ 2014 PACE.pptx
Conclusion
In the pro-inflammatory environment following an ACS, CETP inhibition with dalcetrapib significantly increases HDL-c and apolipoprotein A1 concentrations, as well as in vitro cholesterol efflux. This study suggests that CETP inhibition increases functional HDL-c levels, which is capable of cholesterol efflux through non-ABCA1-mediated pathways. Patient-specific characters appear to influence the extent to which lipids, apolipoproteins and HDL-mediated efflux are altered.Since the improvement of cholesterol efflux function was more modest than the increase in HDL levels, the data further support the hypothesis that there is a disconnect between HDL particle function, HDL-c concentration and CV outcomes, and the correlation between change in cholesterol efflux and CV events warrant further analysis of the dal-OUTCOMES study.
Find this article online
References
1. Kontush A, Chapman MJ. Antiatherogenic small, dense HDL: guardian angel of the arterial wall?. Nat Clin Pract Cardiovasc Med 2006;3:144–153.
2. Asztalos BF, Tani M, Schaefer EJ. Metabolic and functional relevance of HDL subspecies. Curr Opin Lipidol 2011;22:176–185.
3. Khera AV, Cuchel M, Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis.N Engl J Med 2011;364:127–135.
4. Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089–2099.
5. Navab M, Reddy ST, Van Lenten BJ, et al. The role of dysfunctional HDL in atherosclerosis. J Lipid Res 2009;50(Suppl):S145–S149.
x
