CETP inhibition lowers atherogenic lipoprotein concentrations in heterozygous FH
More patients on anacetrapib than on placebo reached LDL-c treatment targets at week 52, while concentrations of particles with potential antiatherogenic properties were increased.
Anacetrapib as lipid-modifying therapy in patients with heterozygous familial hypercholesterolaemia (REALIZE): a randomised, double-blind, placebo-controlled, phase 3 studyLiterature - Kastelein JJ et al., Lancet. 2015
Kastelein JJ, Besseling J, Shah S, et al.
Lancet. 2015 Mar 2. doi: 10.1016/S0140-6736(14)62115-2. [Epub ahead of print]
Background
As a consequence of mutations in the genes encoding the LDL receptor (LDLR), apolipoprotein (apo) B (APOB) or proprotein convertase subtilisin/kexin type 9 (PCSK9), patients with familial hypercholesterolaemia (FH) have high plasma LDL-c concentrations and high cardiovascular (CV) risk [1,2].Although statins have been shown to reduce LDL-c levels and increase life expectancy in patients with heterozygous FH (HeFH), most HeFH patients do not meet recommended LDL-c targets [3].
Cholesterol ester-transfer protein (CETP) inhibitors, like anacetrapib, limit transfer of cholesterol from HDL to LDL particles in plasma [4], which lowers the levels of cholesterol in atherogenic lipoproteins, while HDL-c and apo A1 concentrations increase [5].
The randomised evaluation of anacetrapib lipid-modifying therapy in patients with heterozygous familial hypercholesterolaemia (REALIZE) study assessed the safety and efficacy profile of anacetrapib 100 mg once daily in patients with HeFH, who were on optimum dose of statin and possibly on one or more other lipid-modifying drugs. Patients had a genotype-confirmed or a clinical diagnosis of HeFH. 204 patients were randomised to 52 weeks of treatment with anacetrapib 100 mg (174 completed the 52 weeks), and 102 to placebo (88 completed).
Main results
- Anacetrapib treatment was associated with greater LDL-c reductions from baseline at week 52 as compared to placebo treatment (placebo-adjusted difference: -39.7%, 95%CI: -45.7 to -33.7, P<0.0001).
- Near-maximum reductions in LDL-c were seen during the first 6 weeks of anacetrapib treatment, which were sustained throughout the treatment phase.
- More patients treated with anacetrapib achieved LDL-c < 2.59 mmol/L (135/165 (82%) vs. 15/85 (18%), P<0.0001) and <1.81 mmol?L (72/165 (44%) vs. 4/85 (5%), P<0.0001) at week 52.
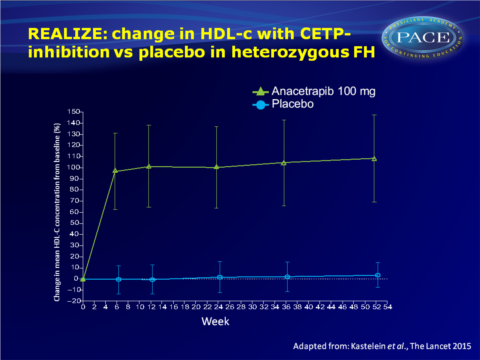
- HDL-c and Apo A1 were significantly elevated with anacetrapib as compared with placebo. The HDL-c increasing effect was near-maximum within 6 weeks, and stable throughout the study.
- Anacetrapib 100 mg was well tolerated, with the only significantly different safety variable being higher diastolic blood pressure in the placebo group. One non-fatal myocardial infarction and three episodes of unstable angina occurred in the anacetrapib group, but this difference was not statistically significant, as was the higher proportion of patients on anacetrapib with adverse events (AEs) leading to discontinuation.
An increased incidence of skin-related AEs was seen with anacetrapib as compared with placebo (19.203 (19%) vs. 2/102 (2%), P=0.0162).
Download Kastelein Realize Lancet 2015 PACE.pptx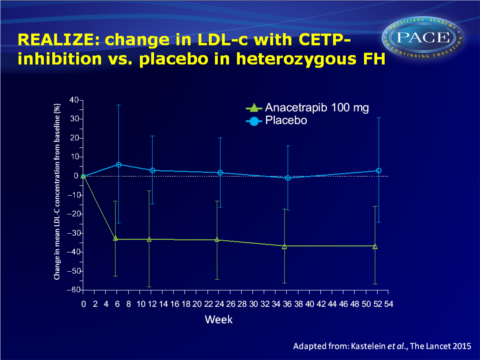
Conclusion
Treatment with anacetrapib 100 mg once daily, in addition to optimal lipid-lowering treatment, lowered plasma concentrations of atherogenic lipoproteins in patients with heFH, while concentrations of particles with potential antiatherogenic properties were increased. More patients on anacetrapib reached LDL-c treatment targets than patients on placebo.Anacetrapib was generally well-tolerated, and, importajntly, did not affect blood pressure.
The effect of CETP inhibition with anacetrapib on CV events needs further assessment in the better-powered REVEAL Study, evaluating anacetrapib in high-risk patients without FH.
Find this article at The Lancet
References
1 Huijgen R, Kindt I, Defesche JC, Kastelein JJ. Cardiovascular risk in relation to functionality of sequence variants in the gene coding for the low-density lipoprotein receptor: a study among 29 365 individuals tested for 64 specifi c low-density lipoprotein-receptor sequence variants. Eur Heart J 2012; 33: 2325–30.
2 Neil A, Cooper J, Betteridge J, et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J 2008; 29: 2625–33.
3 Pijlman AH, Huijgen R, Verhagen SN, et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis 2010; 209: 189–94.
4 Gutstein DE, Krishna R, Johns D, et al. Anacetrapib, a novel CETP inhibitor: pursuing a new approach to cardiovascular risk reduction. Clin Pharmacol Ther 2012; 91: 109–22.
5 Cannon CP, Shah S, Dansky HM, et al, for the Determining the Efficacy and Tolerability Investigators. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 2010; 363: 2406–15.
