CETP inhibition resulted in increased preBeta-1 HDL and cholesterol efflux
CETP inhibitor TA-8995 resulted in non-ABCA1- and ABCA1-specific cholesterol efflux and contemporary preBeta-1 HDL increase.
Effects of the Cholesteryl Ester Transfer Protein Inhibitor, TA-8995, on Cholesterol Efflux Capacity and HDL Particle SubclassesLiterature - Van Capelleveen JC et al., J Clin Lipidol 2016
Van Capelleveen JC, Kastelein JJP, Zwinderman AH, et al.
J Clin Lipidol 2016; Pub ahead of print
Background
Cholesteryl ester transfer protein (CETP) transfers cholesteryl esters from HDL to apoB-containing lipoproteins. CETP inhibitors were developed to raise HDL-c levels, in the assumption that this would result in cardiovascular disease risk reduction as HDL removes excess of cholesterol from the blood. In this process, different acceptors that transfer cholesterol to specific HDL particles are involved to promote cholesterol efflux. For example, ATP binding cassette transporter A1 (ABCA1) transfers cellular free-cholesterol to small nascent preBeta-1 HDL particles.Although CETP inhibitors increase HDL-c and apoA-1 levels, the effect size of the different CETP inhibitors on HDL-c and apoA-I levels varies greatly [1-4]. Furthermore, CVD outcome studies failed to show beneficial effect [1,2]. This observation initiated a debate on the functionality of the HDL that is generated by CETP inhibitors and it has been speculated that CETP inhibitors may promote large non-functional HDL particles [5].
In this study, CETP inhibitor TA-8995 was investigated for its cholesterol efflux capacity (CEC) of plasma from CETP-inhibitor-treated patients who were enrolled in the TULIP trial. The effects on non-ABCA1- and ABCA1-specific CEC and changes in HDL subparticle distribution were investigated. CEC was measured in fasted plasma samples at baseline and week 12 and 37 patients were randomised to placebo, 37 to 1mg TA-8995, 39 to 5mg TA-8995, 35 to 10mg TA-8995 and 39 to combined 10mg TA-8995 with 10mg rosuvastatin.
Main results
- CEC increased dose-dependently (17%, 33%, 38% for patients receiving 1, 5, 10mg TA-8995 respectively). CEC increased 31% in patients with combined treatment (all P<0.001 compared to placebo).
- Non-ABCA1 specific CEC increased dose-dependently (38%, 62%, 72% for patients receiving 1, 5, 10mg TA-8995 respectively). Non-ABCA1 specific CEC increased 67% in patients with combined treatment (all P<0.001 compared to placebo).
- ABCA1-specific CEC increased dose-dependently (14%, 25%, 28% for patients receiving 1, 5, 10mg TA-8995 respectively, P<0.05 for 5mg and P<0.01 for 10mg). ABCA1-specific CEC increased 16% in patients with combined treatment (P=NS compared to placebo).
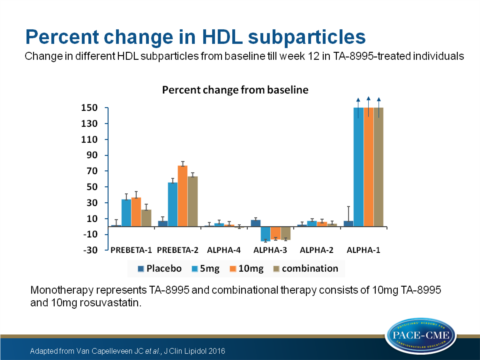
- Large alpha-1 HDL particle levels increased by 350% and 352% (P<0.001) in patients receiving mono- (all doses combined in this analysis) and combination therapy, respectively. Alpha-2 and alpha-4 were not significantly changed, whereas alpha-3 HDL was decreased by 17% from baseline in both mono- and combinational therapy groups (P<0.001).
- PreBeta-1 HDL was significantly increased by 36% for (in this analysis combined doses) TA-8995 monotherapy (P<0.001) and 22% for combined therapy (P=NS).
- PreBeta-2 HDL increased by 66% for patients with monotherapy and 64% for patients receiving combined therapy (P<0.001).
- HDL-c increase correlated with non-ABCA1 specific CEC increase (R2 change 0.08, P<0.001).
- PreBeta-1 HDL increase correlated with total-and ABCA1 specific CEC increase (R2 change 0.16 and 0.15 respectively, P<0.001) and with non-ABCA1 specific CEC (R2 change 0.04, P<0.05).
- Baseline total CEC was largely based on HDL-c and preBeta-1 HDL levels.
Download ~$Van Capelleveen 2016 PACE.pptx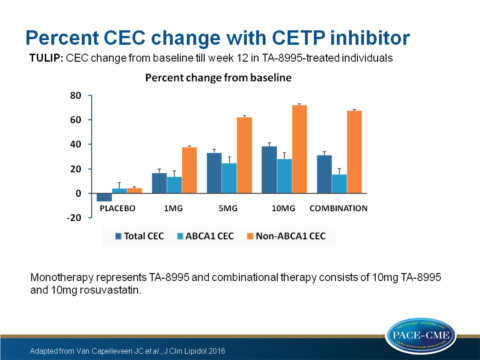
Conclusion
Treatment with the CETP inhibitor TA-8995 resulted in an increase of total cholesterol efflux capacity (CEC), which was non-ABCA1 specific as well as ABCA1 specific CEC. Furthermore, treatment resulted in increased preBeta-1 HDL levels. In contrast to HDL-c increase, preBeta-1 HDL increase was an important predictor of ABCA1- and total CEC increase.Find this article online at J Clin Lipidol
References
1. Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk 27 for coronary events. N Engl J Med. 2007;357(21):2109-2122. 28 doi:10.1056/NEJMoa0706628. 29
2. Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent 30 acute coronary syndrome. N Engl J Med. 2012;367(22):2089-2099. 31 doi:10.1056/NEJMoa1206797.
3. Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high 21 risk for coronary heart disease. N Engl J Med. 2010;363(25):2406-2415. 22 doi:10.1056/NEJMoa1009744. 23 4. Nicholls SJ, Brewer HB, Kastelein JJP, et al. Effects of the CETP inhibitor evacetrapib 24 administered as monotherapy or in combination with statins on HDL and LDL 25 cholesterol: a randomized controlled trial. JAMA. 2011;306(19):2099-2109. 26 doi:10.1001/jama.2011.1649. 27 15. 5. Brewer HB. Increasing HDL Cholesterol Levels. N Engl J Med. 2004;350(15):1491- 28 1494. doi:10.1056/NEJMp048023.
