Cholesterol efflux capacity inversely associated with incident coronary heart disease events
Large-scale prospective population-based study confirms association between cholesterol efflux capacity and coronary heart disease, independent of established CV risk factors.
Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control studyLiterature - Saleheen D et al., Lancet Diabetes Endocrinol. 2015
Saleheen D, Scott R, Javad S et al.,
Lancet Diabetes Endocrinol. 2015 May 26. doi: 10.1016/S2213-8587(15)00126-6
Background
Preclinical studies have shown direct antiatherogenic effects of HDL-c or its major protein apoA-I, including prevention or regression of lesions in animals models of atherosclerosis [1,2]. Clinical trials and genetic variation studies, however, do not confirm that scenarios that raise HDL-c levels decrease risk of coronary heart disease (CHD). Therefore, effort is now directed at studying different properties of HDL.Cholesterol efflux capacity reflects the ability of HDL to promote cholesterol removal from lipid-laden macrophages, which is the first step in reverse cholesterol transport [3]. HDL cholesterol efflux capacity was found to be inversely associated with prevalent risk of CHD in a cross-sectional study [4], but this study design has inherent limitations. A subsequent report confirmed an inverse relationship with prevalent CHD, but suggested the potential for a positive association between cholesterol efflux capacity with incident CV events [5]. In a small cohort study, efflux capacity was inversely associated with incident CV disease events, independent of HDL-c levels [6].
This is an analysis of the association between HDL-c efflux capacity and incident CHD in a prospective, population-based nested case-control study with patients from the EPIC-Norfolk study [7] in the UK. 1745 initially healthy people who later developed fatal or non-fatal CHD were identified, and matched to initially healthy people who did not develop any CV disease during follow-up.
Main results
- In control participants, cholesterol efflux capacity was positively correlated with HDL-c levels (r=0.40, 95%CI: 0.36-0.42) and apoA-1 (r=0.22, 95%CI: 0.18-0.26), and less so with total cholesterol (r=0.18) and LDL-c (r=0.05), as well as female sex (r=0.24) and alcohol intake (r=0.12), while inverse associations with waist:hip ratio, BMI and history of diabetes were seen.
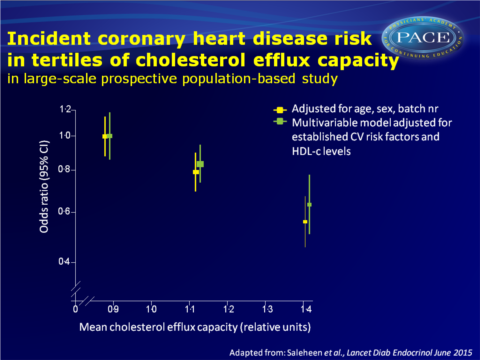
- OR (adjusted for age, sex and batch number) for CHD was 0.56 (95%CI: 0.46-0.62) when comparing individuals with cholesterol efflux capacity in the highest vs. the bottom tertile.
Also after progressive adjustment for various CV risk factors, cholesterol efflux capacity was significantly associated with incident CHD events. - OR for CHD per-SD increase in cholesterol efflux capacity was 0.80 (95%CI: 0.70-0.90) in the final multivariable model adjusted for HDL-concentration. ORs for CHD progressively decreased across tertiles of efflux capacity.
- Using apoA-1 concentrations instead of HDL-c levels for adjustment, did not change the association between efflux capacity and CHD events.
- When comparing top vs. bottom tertiles of HDL-c or apoA-1 levels, they were both inversely associated with CHD events, independently of CV risk factors, but these associations were non-significant after further adjustment for cholesterol efflux capacity.
- No interactions were seen between the association of efflux capacity and CHD and specific subgroups of CV risk factors, although there was a trend towards a stronger association in men vs women and in never smokers vs. ever smokers.
Download Saleheen Lancet Diabetes 2015 PACE.pptx
Conclusion
This large-scale prospective population-based study shows that cholesterol efflux capacity was significantly and inversely associated with incident coronary heart disease events, independent of established CV risk factors, including HDL-c and apoA-I concentrations. Because the causal nature of the relationship between efflux capacity and CHD risk is uncertain as yet, studies considering genetic information need to exclude residual confounding.Find this article online at Lancet Diabetes Endocrinol
References
1 Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest 1990; 85: 1234–41.
2 Tangirala RK, Tsukamoto K, Chun SH, et al. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation 1999; 100: 1816–22.
3 Rothblat GH, de la Llera-Moya M, Atger V, et al. Cell cholesterol effl ux: integration of old and new observations provides new insights. J Lipid Res 1999; 40: 781–96.
4 Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011; 364: 127–35.
5 Li XM, Tang WH, Mosior MK, et al. Paradoxical association of enhanced cholesterol effl ux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol 2013; 33: 1696–705.
6 Rohatgi A, Khera A, Berry JD, et al. HDL Cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014; 371: 2383–93.
7 Day N, Oakes S, Luben R, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 1999; 80 (suppl 1): 95–103.
