Clinically guided dose lowering of FXa-inhibitor can optimise risk-benefit balance of anticoagulation
Analysis of ENGAGE AF-TIMI 48 shows that the efficacy of edoxaban as compared with warfarin is preserved when the dose is reduced based on clinical features, without routine monitoring of drug concentration or anticoagulant activity.
Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trialLiterature - Ruff CT et al., Lancet. 2015
Ruff CT, Giugliano RP, Braunwald E, et al.
Lancet. 2015 Mar 10. doi: 10.1016/S0140-6736(14)61943-7. [Epub ahead of print]
Background
Several new or non-vitamin K antagonist (VKA) oral anticoagulants (NOACs) that directly inhibit thrombin or activated factor Xa (FXa) have been found to be at least as effective and safer than warfarin in preventing stroke and systemic embolic events (SE) in patients with atrial fibrillation (AF). Because of their more predictable anticoagulant effect than that of VKA, they are administered in fixed doses, without the need for routine coagulation monitoring.A pharmacokinetic analysis from the RE-LY trial showed, however, that fixed dosing of the thrombin inhibitor dabigatran resulted in significant variation in plasma concentrations. This depended on patient factors, for instance older age and renal dysfunction were associated with higher dabigatran concentrations and the risk of bleeding [1]. Thus, measurement of drug concentration or anticoagulant activity may be needed when prescribing NOACs.
The ENGAGE AF-TIMI 48 trial evaluated two once-daily dose regimens of the FXa inhibitor edoxaban in comparison with warfarin in over 21000 patients with AF at moderate-high stroke risk [2,3]. Both edoxaban regimens were non-inferior to warfarin in the prevention of stroke or SE, and reduced major bleeding, intracranial haemorrhage and CV mortality [2]. A 50% dose reduction was applied in patients with clinical features known to increase drug exposure and the risk of bleeding.
This analysis aimed to assess whether tailoring of the dose of NOACs on the basis of clinical information alone prevented excess drug levels and whether this optimised the balance between the risk of ischaemic and bleeding events in the ENGAGE AF-TIMI 48 trial.
Main results
- In 2865 patients randomised to edoxaban, trough anti-FXa activity was measured and related to trough mean of edoxaban concentration. A four-fold range of edoxaban dosing (15-60 mg) was associated with a 3-fold gradient of the trough mean of edoxaban concentration and a 2.4-fold gradient of mean anti-FXa activity, with largely overlapping dose groups. Edoxaban concentration and anti-FxA activity were highly correlated (r=0.96, 95%CI: 0.95-0.96, P<0.0001).
- Patients who qualified for dose reduction (including of placebo) at randomisation (n=5356, n=25.4%), had higher rates of thromboembolic events, bleeding and death as compared with patients who did not meet criteria for dose reduction.
Patients randomly assigned to higher-dose edoxaban who had a dose reduction, had higher rates of stroke or SE and major bleeding than those randomised to lower-dose edoxaban and who did not have a dose reduction. - Decreased edoxaban exposure and anti-FXa activity through dose-reduction did not alter the efficacy to prevent stroke or SE, or ischaemic stroke and all-cause mortality as compared with warfarin. In the lower-dose regimen, a greater reduction in fatal bleeding and intracranial haemorrhage was seen upon dose reduction, as compared with warfarin.
- An exploratory analysis suggested that efficacy of edoxaban was preserved in patients who had a dose reduction due to renal dysfunction or bodyweight of <60 kg. In the <10% of patients in which dose was adjusted due to concomitant medication with potent P-glycoprotein interaction, a relative increase in stroke/SE risk was seen with adjusted dose edoxaban (28 events).
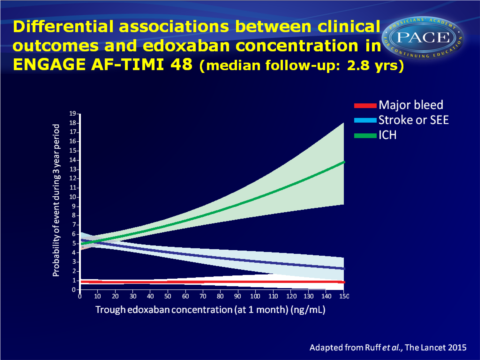
- Differential associations between edoxaban concentration and various safety and efficacy outcomes were observed. The association of intracranial haemorrhage was a relatively flat association across edoxaban concentrations, and this was an infrequent event.
Download RUFF Lancet 2015 PACE.pptx
Conclusion
This analysis of edoxaban concentration, anti-FXA activity, efficacy and bleeding outcomes, shows that the efficacy of edoxaban as compared with warfarin was preserved in patients in whom the dose was reduced, even though patients who qualified for dose reduction had higher rates of ischaemic and bleeding events (including patients who had a dose reduction of placebo). The reduction of major bleeding was even greater in case of dose reduction of edoxaban as compared with warfarin.The data suggest that relying on concentration or coagulation testing alone can be misleading, since patients who had dose reductions and lower drug concentrations and anti-FXa activity, generally had higher rates of bleeding and ischaemic events than in patients who did not have a dose reduction. These findings validate a strategy of adjusting the dose of edoxaban based on clinical features without routine measurement of drug concentration or anticoagulant activity.
Editorial comment [4]
“…plotting the therapeutic probability of clinical events (ie, intracerebral haemorrhage, major bleeding, and stroke or systemic embolism) against edoxaban concentrations, the investigators conclude that dose reduction preserved the clinical effect and that the therapeutic window for bleeding is less than that for protection against stroke or systemic embolism.” (…) “They note that patients with atrial fibrillation and renal dysfunction, the main driver for dose reduction, have a significantly higher risk of stroke and bleeding than those without renal dysfunction.” (…) “The plot of edoxaban trough concentrations and clinical outcomes in ENGAGE AF TIMI-48 shows that intracerebral haemorrhage has a weak correlation with edoxaban concentration, pointing towards a specific warfarin-dependent hazard for this important clinical endpoint. These data probably explain the consistent benefit with regard to intracranial haemorrhage seen across all the NOACs. The plot for stroke and systemic embolism shows a broad concentration range for reduction, whereas major bleeding correlated the closest with edoxaban concentration but has variability most likely because of patient factors.”Find this article online at The Lancet
References
1 Reilly PA, Lehr T, Haertter S, et al, and the RE-LY Investigators. The eff ect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (randomized evaluation of long-Term anticoagulation therapy). J Am Coll Cardiol 2014; 63: 321–28.
2 Giugliano RP, Ruff CT, Braunwald E, et al, and the ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093–104.
3 Ruff CT, Giugliano RP, Antman EM, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the effective anticoagulation with factor xa next generation in atrial fibrillation-thrombolysis in myocardial infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J 2010; 160: 635–41.
4 Patel MR and Washam JF. Edoxaban and the need for outcomes-based NOAC dosing. Published online. March 11, 2015 http://dx.doi.org/10.1016/S0140-6736(14)62289-3
