Combination therapy for type 2 diabetes better in teenagers
02/05/2012
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study assessed how to manage diabetes in children and adolescents. A combination of metformin plus rosiglitazone is more effective in treating youth with type 2 diabetes than metformin alone
A Clinical Trial to Maintain Glycemic Control in Youth with Type 2 Diabetes.Literature - TODAY Study Group, N Engl J Med. 2012 Apr 29
TODAY Study Group.
N Engl J Med. 2012 Apr 29.
The incidence of type 2 diabetes in young people has increased due to an increase in childhood obesity [1,2]. Achieving and sustaining metabolic control is important to reduce the risk of micro- and macrovascular complications in adulthood.
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study assessed how to manage diabetes in children and adolescents. The study involved 699 youth 10 to 17 years of age diagnosed with type 2 diabetes within the previous 2 years, with a body mass index (BMI) in the 85th percentile or above, and without pancreatic autoimmunity. The average BMI of the study participants was in the 98th percentile. The participants were randomly assigned to receive either metformin alone, metformin plus rosiglitazone, or metformin along with an intensive diet and exercise regimen. The primary end point was time to failure of glycemic control, defined as a glycated hemoglobin value of 8.0% or greater for at least 165 days or of 10.0% or greater at the end of the study. Participants were followed for 2 to 6 years.
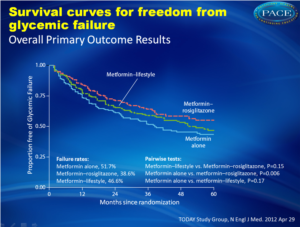
Over an average follow-up of 46 months, 51.7% of the children taking metformin alone experienced failure to control long-term blood glucose levels, with median time to failure of 11.8 months. Adding rosiglitazone reduced the failure rate to 38.6% — a reduction of 25% over metformin alone (P=0.006), with median time to failure of 10.3 months. The failure rate for metformin plus lifestyle intervention was 46.6% (P=0.17 vs metformin alone), with median time to failure of 12.0 months. The differences in time to failure were not significant.
Because of evidence linking rosiglitazone, a thiazolidinedione, to an increased risk for heart attacks and strokes in adults, the US Food and Drug Administration restricted its use in September 2010. In examining the safety of all participants, the TODAY Data Safety and Monitoring Board recommended that the trial continue to test rosiglitazone
1. Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 2005;146:693-700.
2. Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000;136:664-72.
Despite the increasing prevalence of type 2 diabetes in youth, there are few data to guide treatment. We compared the efficacy of three treatment regimens to achieve durable glycemic control in children and adolescents with recent-onset type 2 diabetes.
Methods
Eligible patients 10 to 17 years of age were treated with metformin (at a dose of 1000 mg twice daily) to attain a glycated hemoglobin level of less than 8% and were randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg twice a day) or a lifestyle-intervention program focusing on weight loss through eating and activity behaviors. The primary outcome was loss of glycemic control, defined as a glycated hemoglobin level of at least 8% for 6 months or sustained metabolic decompensation requiring insulin.
Results
Of the 699 randomly assigned participants (mean duration of diagnosed type 2 diabetes, 7.8 months), 319 (45.6%) reached the primary outcome over an average follow-up of 3.86 years. Rates of failure were 51.7% (120 of 232 participants), 38.6% (90 of 233), and 46.6% (109 of 234) for metformin alone, metformin plus rosiglitazone, and metformin plus lifestyle intervention, respectively. Metformin plus rosiglitazone was superior to metformin alone (P=0.006); metformin plus lifestyle intervention was intermediate but not significantly different from metformin alone or metformin plus rosiglitazone. Prespecified analyses according to sex and race or ethnic group showed differences in sustained effectiveness, with metformin alone least effective in non-Hispanic black participants and metformin plus rosiglitazone most effective in girls. Serious adverse events were reported in 19.2% of participants.
Conclusions
Monotherapy with metformin was associated with durable glycemic control in approximately half of children and adolescents with type 2 diabetes. The addition of rosiglitazone, but not an intensive lifestyle intervention, was superior to metformin alone.
N Engl J Med. 2012 Apr 29.
Background
The incidence of type 2 diabetes in young people has increased due to an increase in childhood obesity [1,2]. Achieving and sustaining metabolic control is important to reduce the risk of micro- and macrovascular complications in adulthood.The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study assessed how to manage diabetes in children and adolescents. The study involved 699 youth 10 to 17 years of age diagnosed with type 2 diabetes within the previous 2 years, with a body mass index (BMI) in the 85th percentile or above, and without pancreatic autoimmunity. The average BMI of the study participants was in the 98th percentile. The participants were randomly assigned to receive either metformin alone, metformin plus rosiglitazone, or metformin along with an intensive diet and exercise regimen. The primary end point was time to failure of glycemic control, defined as a glycated hemoglobin value of 8.0% or greater for at least 165 days or of 10.0% or greater at the end of the study. Participants were followed for 2 to 6 years.
Main results
Over an average follow-up of 46 months, 51.7% of the children taking metformin alone experienced failure to control long-term blood glucose levels, with median time to failure of 11.8 months. Adding rosiglitazone reduced the failure rate to 38.6% — a reduction of 25% over metformin alone (P=0.006), with median time to failure of 10.3 months. The failure rate for metformin plus lifestyle intervention was 46.6% (P=0.17 vs metformin alone), with median time to failure of 12.0 months. The differences in time to failure were not significant.Conclusion
A combination of metformin plus rosiglitazone is more effective in treating youth with type 2 diabetes than metformin alone. Adding an intensive lifestyle intervention to metformin is no more effective than metformin alone.
Note
Because of evidence linking rosiglitazone, a thiazolidinedione, to an increased risk for heart attacks and strokes in adults, the US Food and Drug Administration restricted its use in September 2010. In examining the safety of all participants, the TODAY Data Safety and Monitoring Board recommended that the trial continue to test rosiglitazone
References
1. Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 2005;146:693-700.2. Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000;136:664-72.
Abstract
BackgroundDespite the increasing prevalence of type 2 diabetes in youth, there are few data to guide treatment. We compared the efficacy of three treatment regimens to achieve durable glycemic control in children and adolescents with recent-onset type 2 diabetes.
Methods
Eligible patients 10 to 17 years of age were treated with metformin (at a dose of 1000 mg twice daily) to attain a glycated hemoglobin level of less than 8% and were randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg twice a day) or a lifestyle-intervention program focusing on weight loss through eating and activity behaviors. The primary outcome was loss of glycemic control, defined as a glycated hemoglobin level of at least 8% for 6 months or sustained metabolic decompensation requiring insulin.
Results
Of the 699 randomly assigned participants (mean duration of diagnosed type 2 diabetes, 7.8 months), 319 (45.6%) reached the primary outcome over an average follow-up of 3.86 years. Rates of failure were 51.7% (120 of 232 participants), 38.6% (90 of 233), and 46.6% (109 of 234) for metformin alone, metformin plus rosiglitazone, and metformin plus lifestyle intervention, respectively. Metformin plus rosiglitazone was superior to metformin alone (P=0.006); metformin plus lifestyle intervention was intermediate but not significantly different from metformin alone or metformin plus rosiglitazone. Prespecified analyses according to sex and race or ethnic group showed differences in sustained effectiveness, with metformin alone least effective in non-Hispanic black participants and metformin plus rosiglitazone most effective in girls. Serious adverse events were reported in 19.2% of participants.
Conclusions
Monotherapy with metformin was associated with durable glycemic control in approximately half of children and adolescents with type 2 diabetes. The addition of rosiglitazone, but not an intensive lifestyle intervention, was superior to metformin alone.