Complementary effects ezetimibe on potent statins
05/11/2012
This study evaluated effects of ezetimibe added to different statin therapies on lowering LDL-C and on markers of cholesterol absorption and synthesis.
Effects of ezetimibe added to statin therapy on markers of cholesterol absorption and synthesis and LDL-C lowering in hyperlipidemic patients.Literature - Thongtang N et al, Atherosclerosis. 2012;225:388-96 - Atherosclerosis. 2012;225:388-96. doi: 10.1016/j.atherosclerosis.2012.09.001.
Thongtang N, Lin J, Schaefer EJ, Lowe RS, Tomassini JE, Shah AK, Tershakovec AM.
Atherosclerosis. 2012;225:388-96. doi: 10.1016/j.atherosclerosis.2012.09.001.
A substantial number of patients treated with statin therapy still have LDL-C levels above guideline-recommended targets [1-4]. Statins not only have a direct inhibitory effect on cholesterol synthesis, but also reduce markers of cholesterol synthesis, such as lathosterol, which can reveal subsequent increases in markers of cholesterol absorption [5-9], dependent on statin dose and type of statin. Ezetimibe is a selective cholesterol absorption inhibitor blocking transport of cholesterol and phytosterols across the intestinal wall, thereby significantly reducing LDL-C levels by 15-20% [10,11].
Co-administration of ezetimibe together with a statin inhibits cholesterol absorption and synthesis [12], producing significantly greater reductions in LDL-C than either drug alone [11,13,14]. In this post hoc analysis of the EASE (Ezetimibe Add-on to Statin for Effectiveness) study the effects of adding ezetimibe 10 mg to different statins and doses on plasma lipid-lowering effects and non-cholesterol sterol levels were evaluated. Comparison of subjects was based on statin type or potency (low, medium, high) subgroups. The low-potency statin group included subjects receiving simvastatin ≤10 mg/day, lovastatin ≤20 mg/day, pravastatin ≤20 mg/day, and fluvastatin ≤40 mg/day. The medium-potency statin group included subjects receiving simvastatin > 10-≤40 mg/day, atorvastatin ≤ 20 mg/day, lovastatin > 20-80 mg/day, pravastatin > 20-80 mg/day, and fluvastatin > 40-80 mg/day. The high-potency statin group included subjects receiving simvastatin > 40-80 mg/ day, and atorvastatin > 20-80 mg/day.
Lipid and non-cholesterol sterol data from the ezetimibe arm of this study were used to test the hypothesis that ezetimibe, when added to statin therapy, would be most effective in LDL-C lowering in subjects on high-potency statins and that these effects would be related to alterations in markers of cholesterol absorption (b-sitosterol, b-sitosterol/cholesterol) and synthesis (lathosterol, lathosterol/cholesterol).
These results highlight the complementary effects of statins and ezetimibe on modulating markers of cholesterol synthesis and absorption. Patients on (high-potency) statins may be good candidates for ezetimibe therapy if additional LDL-C lowering is required to reach LDL-C goals.
2. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation 2004;110:227e39.
3. Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and the European atherosclerosis society (EAS). Atherosclerosis 2011;217:3e46.
4. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J 2012;33:1635e701.
5. Reihnér E, Rudling M, Stáhlberg D, et al. Influence of pravastatin, a specific inhibitor of HMG-CoA reductase, on hepatic metabolism of cholesterol. N Engl J Med 1990;323:224e8.
6. Lamon-Fava S, Diffenderfer MR, Barrett PH, et al. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J Lipid Res 2007;48:1746e53.
7. Vanhanen H, Miettinen TA. Pravastatin and lovastatin similarly reduce serum cholesterol and its precursor levels in familial hypercholesterolaemia. Eur J Clin Pharmacol 1992;42:127e30.
8. Ooi EM, Barrett PH, Chan DC, Nestel PJ, Watts GF. Dose-dependent effect of rosuvastatin on apolipoprotein B-100 kinetics in the metabolic syndrome. Atherosclerosis 2008;197:139e46.
9. De Cuyper I, Wolthers BG, van Doormaal JJ, Wijnandts PN. Determination of changes in serum lathosterol during treatment with simvastatin to evaluate the role of lathosterol as a parameter for whole body cholesterol synthesis. Clin Chim Acta 1993;219:123e30.
10. Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol 2002;40:2125e34.
11. Gagne C, Gaudet D, Bruckert E. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation 2002;105:2469e75.
12. Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis 2008;198:198e207.
13. Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003;107:2409e15.
14. Morrone D, Weintraub WS, Toth PP, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis 2012;223:251e61.
Objective:
Statins inhibit cholesterol synthesis but can upregulate cholesterol absorption, with higher doses producing larger effects. Ezetimibe inhibits cholesterol absorption but also upregulates synthesis. We tested whether ezetimibe added to on-going statin therapy would be most effective in lowering LDL-cholesterol (LDL-C) in subjects on high-potency statins and whether these effects would be related to alterations in cholesterol absorption (β-sitosterol) and synthesis (lathosterol) markers.
Methods:
Hypercholesterolemic subjects (n = 874) on statins received ezetimibe 10 mg/day. Plasma lipids, lathosterol, and β-sitosterol were measured at baseline and on treatment. Subjects were divided into low- (n = 133), medium- (n = 582), and high- (n = 159) statin potency groups defined by predicted LDL-C-lowering effects of each ongoing statin type and dose (reductions of ∼20-30%, ∼31-45%, or ∼46-55%, respectively).
Results:
The high-potency group had significantly lower baseline lathosterol (1.93 vs. 2.58 vs. 3.17 μmol/l; p < 0.001) and higher baseline β-sitosterol values (6.21 vs. 4.58 vs. 4.51 μmol/l, p < 0.001) than medium-/low-potency groups. Ezetimibe treatment in the high-potency group produced significantly greater reductions from baseline in LDL-C than medium-/low-potency groups (-29.1% vs. -25.0% vs. -22.7%; p < 0.001) when evaluating unadjusted data. These effects and group differences were significantly (p < 0.05) related to greater β-sitosterol reductions and smaller lathosterol increases. However, LDL-C reduction differences between groups were no longer significant after controlling for placebo effects, due mainly to modest LDL-C lowering by placebo in the high-potency group.
Conclusion:
Patients on high-potency statins have the lowest levels of cholesterol synthesis markers and the highest levels of cholesterol absorption markers at baseline, and the greatest reduction in absorption markers and the smallest increases in synthesis markers with ezetimibe addition. Therefore, such patients may be good candidates for ezetimibe therapy if additional LDL-C lowering is needed.
Atherosclerosis. 2012;225:388-96. doi: 10.1016/j.atherosclerosis.2012.09.001.
Background
A substantial number of patients treated with statin therapy still have LDL-C levels above guideline-recommended targets [1-4]. Statins not only have a direct inhibitory effect on cholesterol synthesis, but also reduce markers of cholesterol synthesis, such as lathosterol, which can reveal subsequent increases in markers of cholesterol absorption [5-9], dependent on statin dose and type of statin. Ezetimibe is a selective cholesterol absorption inhibitor blocking transport of cholesterol and phytosterols across the intestinal wall, thereby significantly reducing LDL-C levels by 15-20% [10,11].Co-administration of ezetimibe together with a statin inhibits cholesterol absorption and synthesis [12], producing significantly greater reductions in LDL-C than either drug alone [11,13,14]. In this post hoc analysis of the EASE (Ezetimibe Add-on to Statin for Effectiveness) study the effects of adding ezetimibe 10 mg to different statins and doses on plasma lipid-lowering effects and non-cholesterol sterol levels were evaluated. Comparison of subjects was based on statin type or potency (low, medium, high) subgroups. The low-potency statin group included subjects receiving simvastatin ≤10 mg/day, lovastatin ≤20 mg/day, pravastatin ≤20 mg/day, and fluvastatin ≤40 mg/day. The medium-potency statin group included subjects receiving simvastatin > 10-≤40 mg/day, atorvastatin ≤ 20 mg/day, lovastatin > 20-80 mg/day, pravastatin > 20-80 mg/day, and fluvastatin > 40-80 mg/day. The high-potency statin group included subjects receiving simvastatin > 40-80 mg/ day, and atorvastatin > 20-80 mg/day.
Lipid and non-cholesterol sterol data from the ezetimibe arm of this study were used to test the hypothesis that ezetimibe, when added to statin therapy, would be most effective in LDL-C lowering in subjects on high-potency statins and that these effects would be related to alterations in markers of cholesterol absorption (b-sitosterol, b-sitosterol/cholesterol) and synthesis (lathosterol, lathosterol/cholesterol).
Main results
- Statin type and dose are significantly associated with levels of cholesterol synthesis and absorption markers
- The addition of ezetimibe to all statin types resulted in significant reductions from baseline in total C, LDL-C, non-HDL-C, Apo B, and total C/HDL-C, with no between-type differences (fig. 1).
- Add-on ezetimibe therapy resulted in significant increases in cholesterol synthesis markers and significant reductions in cholesterol absorption markers from baseline for each statin type and statin potency level.
Conclusion
These results highlight the complementary effects of statins and ezetimibe on modulating markers of cholesterol synthesis and absorption. Patients on (high-potency) statins may be good candidates for ezetimibe therapy if additional LDL-C lowering is required to reach LDL-C goals.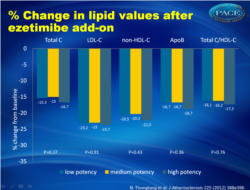 Figure 1.Percent change in lipid values from statin-treated baseline after ezetimibe add-on therapy (adjusted for values from the placebo arm of EASE). |
References
1. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143e421.2. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. Circulation 2004;110:227e39.
3. Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and the European atherosclerosis society (EAS). Atherosclerosis 2011;217:3e46.
4. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J 2012;33:1635e701.
5. Reihnér E, Rudling M, Stáhlberg D, et al. Influence of pravastatin, a specific inhibitor of HMG-CoA reductase, on hepatic metabolism of cholesterol. N Engl J Med 1990;323:224e8.
6. Lamon-Fava S, Diffenderfer MR, Barrett PH, et al. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J Lipid Res 2007;48:1746e53.
7. Vanhanen H, Miettinen TA. Pravastatin and lovastatin similarly reduce serum cholesterol and its precursor levels in familial hypercholesterolaemia. Eur J Clin Pharmacol 1992;42:127e30.
8. Ooi EM, Barrett PH, Chan DC, Nestel PJ, Watts GF. Dose-dependent effect of rosuvastatin on apolipoprotein B-100 kinetics in the metabolic syndrome. Atherosclerosis 2008;197:139e46.
9. De Cuyper I, Wolthers BG, van Doormaal JJ, Wijnandts PN. Determination of changes in serum lathosterol during treatment with simvastatin to evaluate the role of lathosterol as a parameter for whole body cholesterol synthesis. Clin Chim Acta 1993;219:123e30.
10. Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol 2002;40:2125e34.
11. Gagne C, Gaudet D, Bruckert E. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation 2002;105:2469e75.
12. Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis 2008;198:198e207.
13. Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003;107:2409e15.
14. Morrone D, Weintraub WS, Toth PP, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis 2012;223:251e61.
Abstract
Objective:Statins inhibit cholesterol synthesis but can upregulate cholesterol absorption, with higher doses producing larger effects. Ezetimibe inhibits cholesterol absorption but also upregulates synthesis. We tested whether ezetimibe added to on-going statin therapy would be most effective in lowering LDL-cholesterol (LDL-C) in subjects on high-potency statins and whether these effects would be related to alterations in cholesterol absorption (β-sitosterol) and synthesis (lathosterol) markers.
Methods:
Hypercholesterolemic subjects (n = 874) on statins received ezetimibe 10 mg/day. Plasma lipids, lathosterol, and β-sitosterol were measured at baseline and on treatment. Subjects were divided into low- (n = 133), medium- (n = 582), and high- (n = 159) statin potency groups defined by predicted LDL-C-lowering effects of each ongoing statin type and dose (reductions of ∼20-30%, ∼31-45%, or ∼46-55%, respectively).
Results:
The high-potency group had significantly lower baseline lathosterol (1.93 vs. 2.58 vs. 3.17 μmol/l; p < 0.001) and higher baseline β-sitosterol values (6.21 vs. 4.58 vs. 4.51 μmol/l, p < 0.001) than medium-/low-potency groups. Ezetimibe treatment in the high-potency group produced significantly greater reductions from baseline in LDL-C than medium-/low-potency groups (-29.1% vs. -25.0% vs. -22.7%; p < 0.001) when evaluating unadjusted data. These effects and group differences were significantly (p < 0.05) related to greater β-sitosterol reductions and smaller lathosterol increases. However, LDL-C reduction differences between groups were no longer significant after controlling for placebo effects, due mainly to modest LDL-C lowering by placebo in the high-potency group.
Conclusion:
Patients on high-potency statins have the lowest levels of cholesterol synthesis markers and the highest levels of cholesterol absorption markers at baseline, and the greatest reduction in absorption markers and the smallest increases in synthesis markers with ezetimibe addition. Therefore, such patients may be good candidates for ezetimibe therapy if additional LDL-C lowering is needed.
